
US-based biopharmaceutical company TG Therapeutics has begun its Phase II investigator initiated trial of TGR-1202 in combination with ibrutinib to treat relapsed or refractory Diffuse Large B-cell Lymphoma (DLBCL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
TGR-1202 is an orally administered PI3K delta inhibitor programmed to target the delta isoform with nanomolar potency and selectivity over the alpha, beta, and gamma isoforms of PI3K.
The delta isoform of PI3K is expressed in cells of hematopoietic origin and considered responsible for progression and survival of B-cell lymphocytes.
Janssen’s Ibrutinib has been developed as bruton's tyrosine kinase (BTK) inhibitor, which forms a strong covalent bond with BTK to prevent transmission of cell survival signals within the malignant B-cells.
Following the inhibition of BTK protein, ibrutinib helps in killing and reducing the number of cancer cells, thereby delaying cancer progression.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe Phase II trial will be conducted at the University of Nebraska Medical Center (UNMC) and explore the safety and efficacy of combination of TGR-1202 and ibrutinib.
TG Therapeutics executive chairman and Interim CEO Michael Weiss said: “We are excited to launch this study led by Dr Matthew Lunning and Dr Michael Green.
“Dr Lunning has extensive experience treating lymphoma patients with TGR-1202 as a doublet in combination with TG-1101 and also as a triple therapy with ibrutinib.
“While the triple combination appears safe, well tolerated and active, looking at the effects of the all oral doublet combination will expand our understanding of the effects of these agents together and the contribution of TG-1101 in a triple combination.”
The trial will be supported by TG Therapeutics and Janssen Pharmaceuticals who will also share the related expenditure.
Patients are currently being enrolled for the study.
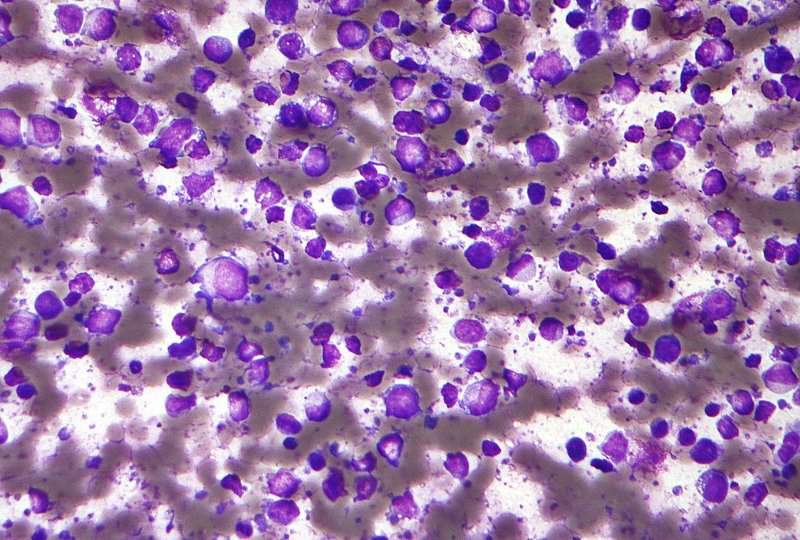
Image: Micrograph of a diffuse large B-cell lymphoma. Photo: courtesy of Nephron.





