
US-based biopharmaceutical company Tyrogenex has completed patient enrolment in the Phase II clinical trial (APEX) of X-82 (vorolanib) for the treatment of wet age-related macular degeneration (wAMD).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
X-82 is an investigational oral agent being developed to inhibit vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR).
The ability of X-82 to bind to these receptors is expected to be efficient in angiogenic diseases such as wAMD.
A total of 157 subjects have been enrolled in the Phase II trial.
The randomised, double-masked, placebo-controlled, dose-finding, non-inferiority APEX trial will evaluate the safety and efficacy of X-82 in the treatment of vision loss due to wAMD.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTyrogenex director and development senior vice-president Daniel Salazar said: "We are grateful for the commitment and enthusiasm of the scientific advisory board and the participating clinical investigators.
"In addition to exceeding our initial enrolment target of 132 patients by 25 patients, we are encouraged that the data safety monitoring board has met three times so far and supported continuing the study unchanged.”
X-82 will be assessed in combination with required intravitreal anti-VEGF, compared to necessary intravitreal anti-VEGF alone in patients with neovascular AMD.
Subjects are being examined for a total of 52 weeks following the administration of one of three doses of the drug or placebo.
X-82 has been investigated in a Phase I trial in patients previously treated with Lucentis (ranibizumab), Eylea (aflibercept) and Avastin (bevacizumab) and in naïve patients, as well as currently being studied for oncology indications.
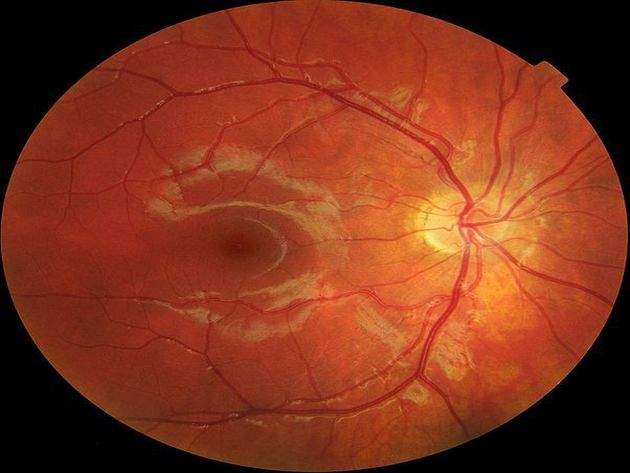
Image: Macular degeneration. Photo: courtesy of Ralf Roletschek/Wikipedia.





