
Researchers at the University of Southampton, UK, have initiated a clinical trial to evaluate Acerta Pharma’s new product candidate acalabrutinib in combination with existing standard therapy called R-CHOP to treat select patients with diffuse large B-cell lymphoma (DLBCL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Acalabrutinib is an inhibitor of a B-cell associated protein called bruton tryrosine kinase (BTK), while R-CHOP is a combination of immunotherapeutic rituximab with four chemotherapy drugs, cyclophosphamide, doxorubicin, vincristine and prednisolone.
Rituximab acts by targeting the CD20 protein present on the surface of lymphoma cells and aids the immune system in finding and destroying them.
Aimed to evaluate the ability of acalabrutinib in improving the response to standard treatments, the new two-part ACCEPT trial will be conducted at seven UK sites.
The trial will include patients aged 16 and older with previously untreated CD20 positive DLBCL.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUniversity of Southampton medical oncology associate professor and consultant Dr Andrew Davies said: “For some lymphoma patients standard treatments are not effective, so we urgently need trials like this to help more people survive their disease.
“Results from previous trials that use acalabrutinib to fight other blood cancers have been very promising. This new and unique drug combination will attack the cancer from two sides.
“Not only will it mark the cancer cells so the immune system can find them and kill them, but it will also prevent the activity of key proteins that play an important role in the spread and survival of malignant B-cells.”
In the first part of the trial, researchers intend to determine a safe and tolerable dose from various low doses of acalabrutinib, while the second part will establish the efficacy of the combination for treating and preventing the disease recurrence.
Samples of the subject’s body fluids such as blood will be analysed at various time points during the trial to monitor the way their body processes acalabrutinib and R-CHOP combination.
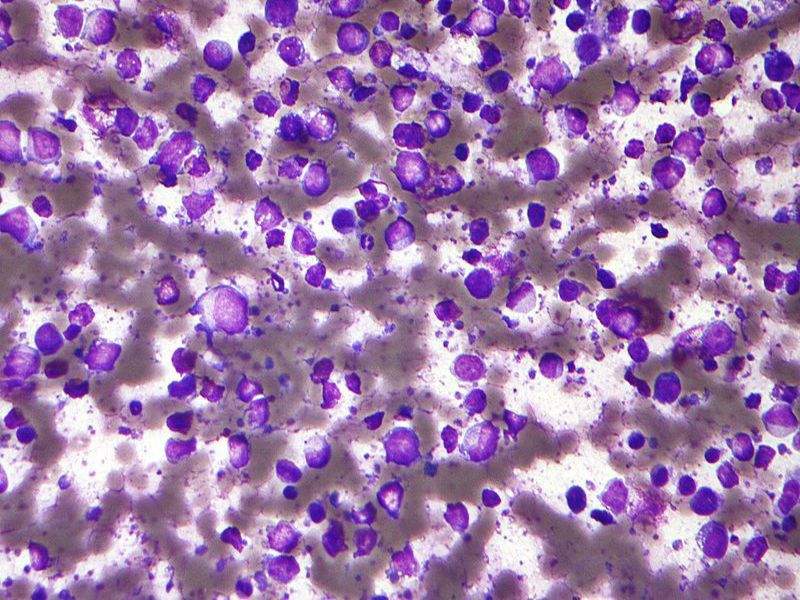
Image: Micrograph of a diffuse large B-cell lymphoma. Photo: courtesy of nephron.





