US-based biotechnology firm Vertex Pharmaceuticals has reported a positive outcome from two Phase III clinical trials of tezacaftor (VX-661) in combination with ivacaftor to treat cystic fibrosis (CF).
Tezacaftor is designed to shift the defective CFTR protein to the proper place in the airway cell surface, while ivacaftor enables the opening of chloride channel on the cell surface to allow movement of chloride and sodium.
The results from EVOLVE and EXPAND trials showed statistically significant improvements in lung function.
The EVOLVE trial met its primary endpoint with a mean absolute improvement in ppFEV1 through 24 weeks, while EXPAND met the primary endpoints of absolute change in ppFEV1 to the average of the week four and week eight measurements.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataSee Also:
Vertex executive vice-president and chief medical officer Jeffrey Chodakewitz said: “The tezacaftor / ivacaftor combination treatment demonstrated clinically meaningful benefits, with a favourable safety profile across multiple patient groups.
“This combination treatment may provide a promising new option for treating the underlying cause of CF in the future and brings us increasingly closer to our goal of developing new medicines for all people with the disease.”
The 24-week, randomised, double-blind, placebo-controlled Phase III EVOLVE trial assessed the efficacy and safety of the combination in 500 patients with two copies of the F508del mutation.
The randomised, double-blind, placebo-controlled, crossover, multi-centre, eight-week Phase III EXPAND trial evaluated the combination's efficacy and safety in 250 patients with one mutation that results in residual CFTR function and one copy of the F508del mutation.
In the third quarter of this year, the firm intends to submit a new drug application (NDA) to the US Food and Drug Administration (FDA) and a marketing authorisation application (MAA) to the European Medicines Agency (EMA) based on these results.
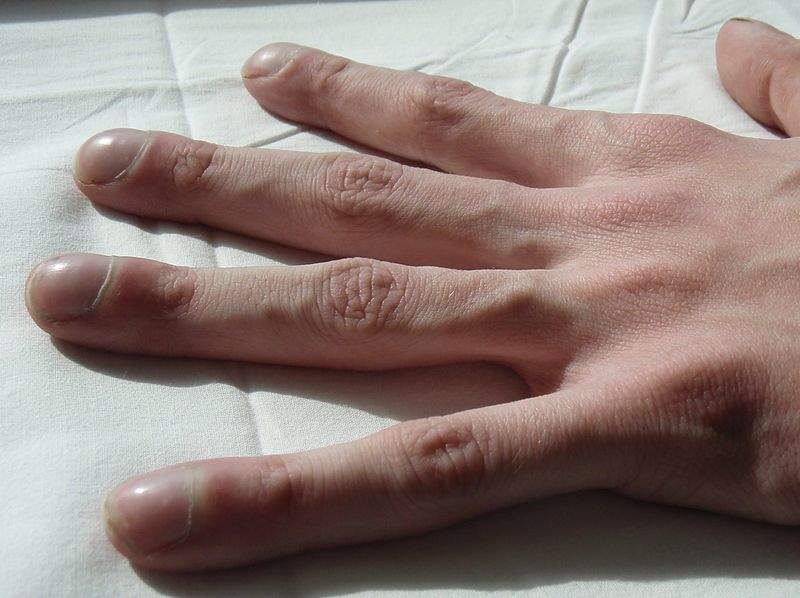
Image: Clubbing of the fingers in a cystic fibrosis patient. Photo: courtesy of Jerry Nick/Wikipedia.