NGM Biopharmaceuticals has reported positive data from the fourth cohort of a Phase II clinical trial of aldafermin in non-alcoholic steatohepatitis (NASH) patients.
Aldafermin (former NGM282) is an engineered version of the FGF19 hormone and is intended to offer a once-daily treatment option for NASH.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The double-blind, randomised, placebo-controlled cohort assessed the safety, efficacy and tolerability of a 1mg dose of the drug over 24 weeks in 78 biopsy-confirmed NASH patients with stage 2 or 3 liver fibrosis (F2-F3).
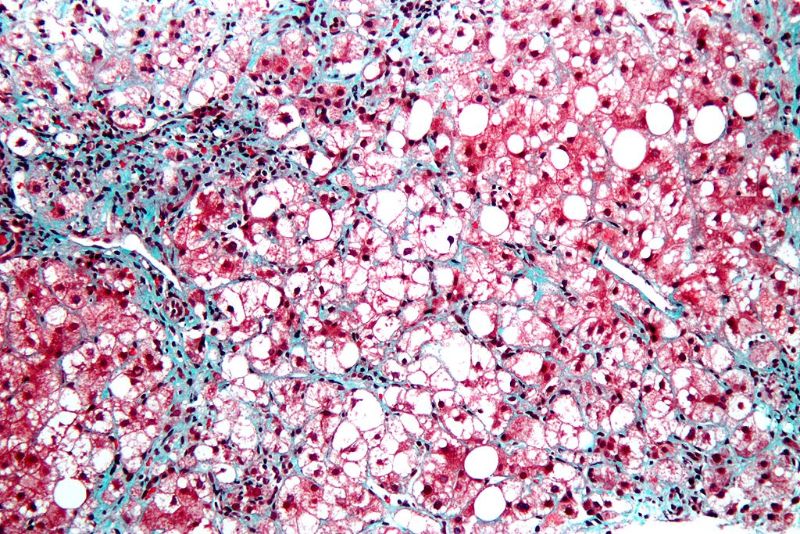
Data showed that the drug led to statistical significance on the primary endpoint of change in absolute liver fat content (LFC), compared to placebo.
Statistical significance was also reached on several secondary and exploratory endpoints of liver histology and disease activity biomarkers.
Histology analysis showed clinically meaningful improvements in fibrosis at 24 weeks in 38% of patients treated with aldafermin versus 18% on placebo. Similarly, NASH resolution was achieved in 24% of patients treated with NGM Bio’s drug compared to 9% treated with placebo.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe drug was generally well-tolerated without any trial discontinuations due to adverse events. The most common adverse events were diarrhoea, headache, nausea, diabetes mellitus, abdominal distension, peripheral oedema and fatigue.
NGM Biopharmaceuticals senior vice-president and chief medical officer Hsiao Lieu said: “We’re very pleased with these data, as they are consistent with the comprehensive body of efficacy and tolerability data generated in over 200 aldafermin-treated NASH patients across our multi-cohort Phase II aldafermin programme.
“Given that aldafermin has a promising effect on fibrosis and NASH resolution and is well-tolerated, we believe this drug could be a central tool in the future treatment landscape of NASH.”
The ongoing Phase IIb ALPINE 2/3 trial is evaluating 0.3mg, 1mg and 3mg doses of aldafermin.





