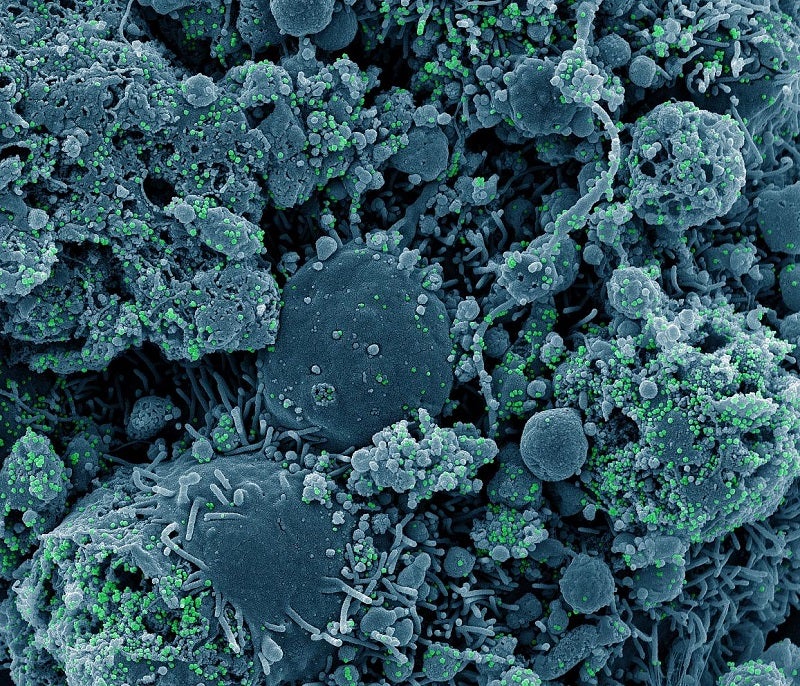
The US National Institute of Allergy and Infectious Diseases (NIAID) researchers have launched a clinical trial for studying systemic allergic reactions in individuals to mRNA vaccines for Covid-19.
Planned to be carried out at a single site, the trial will recruit up to 100 people who are aged 16 to 69 years and experienced an allergic reaction after receiving the first Covid-19 mRNA vaccine dose.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
At the National Institutes of Health’s (NIH) Clinical Center in Bethesda, Maryland, trial subjects will receive a second vaccine dose as inpatients.
NIAID director Anthony Fauci said: “People who experienced an allergic reaction after receiving a Covid-19 mRNA vaccine may be hesitant to complete their vaccine regimen.
“This study will help us determine if individuals who experienced moderate systemic allergic reactions can safely receive a second dose of a Covid-19 mRNA vaccine.”
The trial will enrol subjects who reported a mild or moderate systemic allergic reaction after taking either Pfizer-BioNTech or Moderna’s first mRNA vaccine dose.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHowever, individuals who reported severe allergic reactions to a first Covid-19 mRNA vaccine dose will not be eligible for enrolment in the trial.
The trial is led by Dr Pamela Guerrerio of NIAID Laboratory of Allergic Diseases.
Guerrerio said: “Our study aims to provide a better understanding of the mechanisms responsible for systemic allergic reactions such as hives, swelling, trouble breathing and light-headedness or passing out.”
All trial subjects will be admitted to the NIH Clinical Center’s Intensive Care Unit for a minimum of four days to increase safety.
Randomly each subject will be assigned to receive either Comirnaty, Pfizer’s Covid-19 mRNA vaccine, or an inactive matching placebo dose on consecutive days.
All of them can expect to be vaccinated completely by the time they end their ICU admission.
Furthermore, participants will undergo breathing tests and have frequent blood draws at admission and during the inpatient stay.
Medical staff will use them for identifying details of any allergic or other responses to the Covid-19 vaccine.
At the visit after five months, participants who tolerated the second vaccine dose without any or only mild symptoms will receive the Comirnaty Covid-19 booster vaccine.
In December last year, the Duke Human Vaccine Institute scientists received a contract from NIAID to advance experimental vaccine candidates to production for early clinical trials usage.





