The US National Institutes of Health (NIH) unit National Institute of Allergy and Infectious Diseases (NIAID) has commenced a Phase I clinical trial of an investigational vaccine to prevent Epstein-Barr virus (EBV) infection.
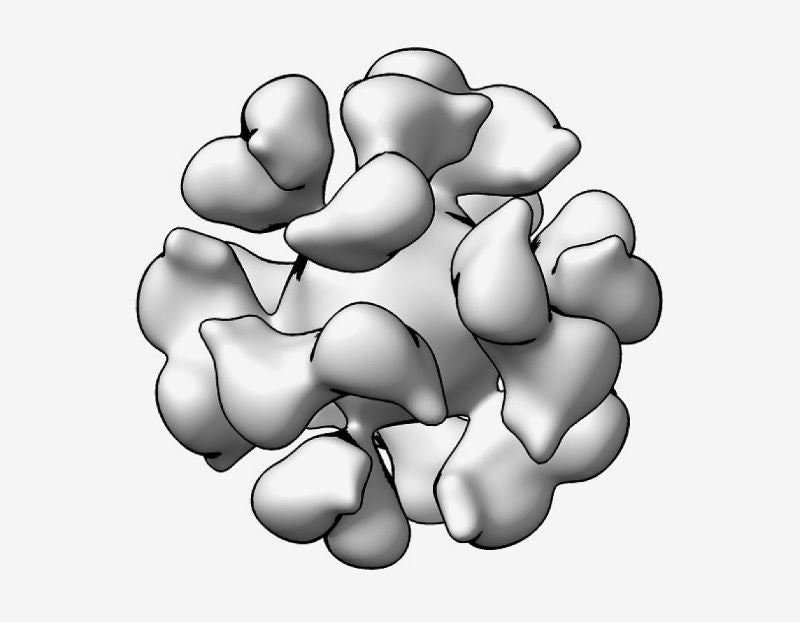
Developed by the Laboratory of Infectious Diseases, in partnership with the Vaccine Research Center of NIAID, the EBV gp350-Ferritin nanoparticle vaccine works by acting on the EBV glycoprotein gp350.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Seen on the surface of the virus and virus-infected cells, EBV gp350 is an initial target for neutralising antibodies observed in naturally infected individuals’ blood.
Ferritin is an iron storage protein in living species’ cells and is regarded as a potential vaccine platform due to its ability to show proteins from the targeted virus in a condensed array on its surface.
To be carried out at the NIH Clinical Center in Bethesda, Maryland, the trial will analyse the safety and immune response of the vaccine, along with Novavax’s saponin-based Matrix-M adjuvant.
Matrix-M adjuvant could possibly boost the immune response elicited by the vaccine.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial will enrol 40 healthy subjects aged 18 to 29 years. Nearly 50% of them should have had previous EBV infections while the rest should not be infected previously.
Trial subjects will be given three 50µg doses of the vaccine in the upper arm muscle.
The second and third doses will be given 30 and 180 days following the preliminary dose.
This trial is one of the two studies to assess an investigational EBV vaccine in more than ten years, NIH noted.
NIAID director Anthony Fauci said: “A vaccine that could prevent or reduce the severity of infection with the Epstein-Barr virus could reduce the incidence of infectious mononucleosis, and might also reduce the incidence of EBV-associated malignancies and autoimmune diseases.”
In November last year, the NIH reported that a combination therapy was beneficial in a Phase III trial for treating standard-risk and high-risk acute promyelocytic leukaemia in children.





