NorthShore University HealthSystem has collaborated with researchers at Johns Hopkins Medicine in the US to carry out Phase II trials of convalescent blood plasma outpatient treatments for Covid-19.
NorthShore is of 30 sites in the US taking part in the trials and is the only site in Illinois.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The US Department of Defense and the National Institutes of Health are funding the research.
At present, only hospitalised Covid-19 patients are given convalescent blood plasma treatment.
NorthShore study lead Giselle Mosnaim said: “We are excited to be a part of this important research.
“These studies will give us the clinical data necessary to determine if convalescent blood plasma therapy can prevent infection and help patients recover more quickly in the early stages of the disease.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe research will aid scientists in better understanding how the immune system protects people from Covid-19, a key point in analysing vaccines.
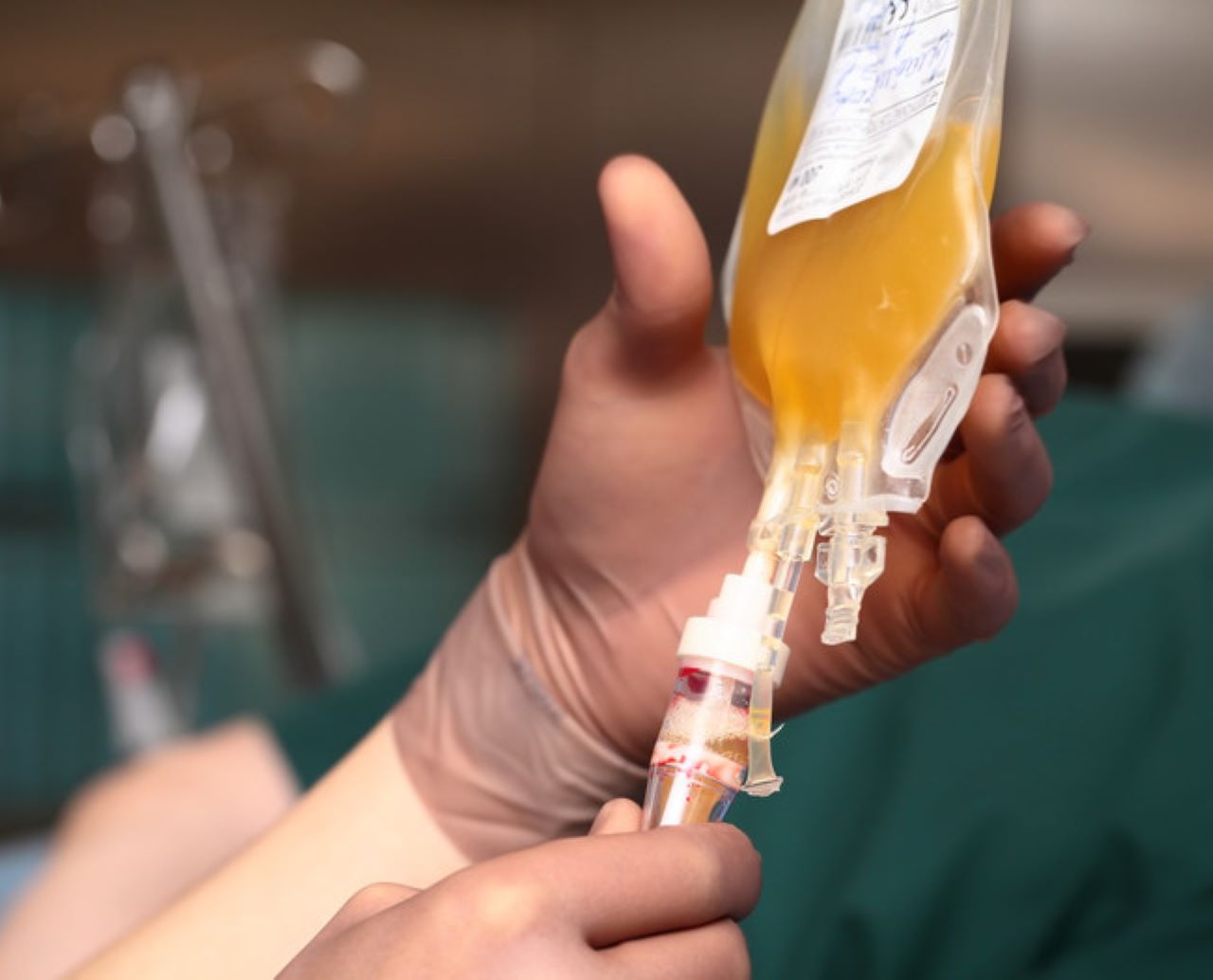
In the convalescent blood plasma therapy, a portion of blood called plasma is transfused from people who have recovered from Covid-19 and showed a strong immune response.
On separating red and white blood cells and platelets in the blood, a yellow-tinged liquid called plasma is obtained and has proteins called antibodies.
These antibodies attach to foreign substances like viruses to mark them for destruction by the immune system or disrupt its ability to multiply and grow.
For both the trials, participants will either be given plasma with or without Covid-19 antibodies as assigned by a computer.
The infection prevention trial plans to enrol 500 adult participants. It will analyse whether giving blood convalescent plasma will prevent illness or reduce the disease severity in people who are at risk of developing Covid-19 after being exposed to the virus.
In the early treatment plasma trial, around 600 adult participants who have early Covid-19 disease will be enrolled and must be 18 or older.
Subjects should be within days of their first symptoms but not ill enough to be hospitalised.





