Northwell Health’s research arm Feinstein Institutes for Medical Research and Cold Spring Harbor Laboratory (CSHL) have initiated recruitment of participants in a fully virtual clinical trial of famotidine for treating adult covid-19 patients in the outpatient setting.
Sold over the counter as PEPCID, famotidine is a histamine 2 blocker to treat heartburn and assist with healing gastrointestinal ulcers.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This is the first time a fully virtual clinical trial was initiated by CSHL and Northwell Health. It has treated over 100,000 Covid-19 patients since the onset of the pandemic in March last year.
The in-home, randomised, double-blind, placebo-controlled trial will enrol Covid-19 patients across the greater New York area who are having mild to moderate symptoms and do not need hospitalisation.
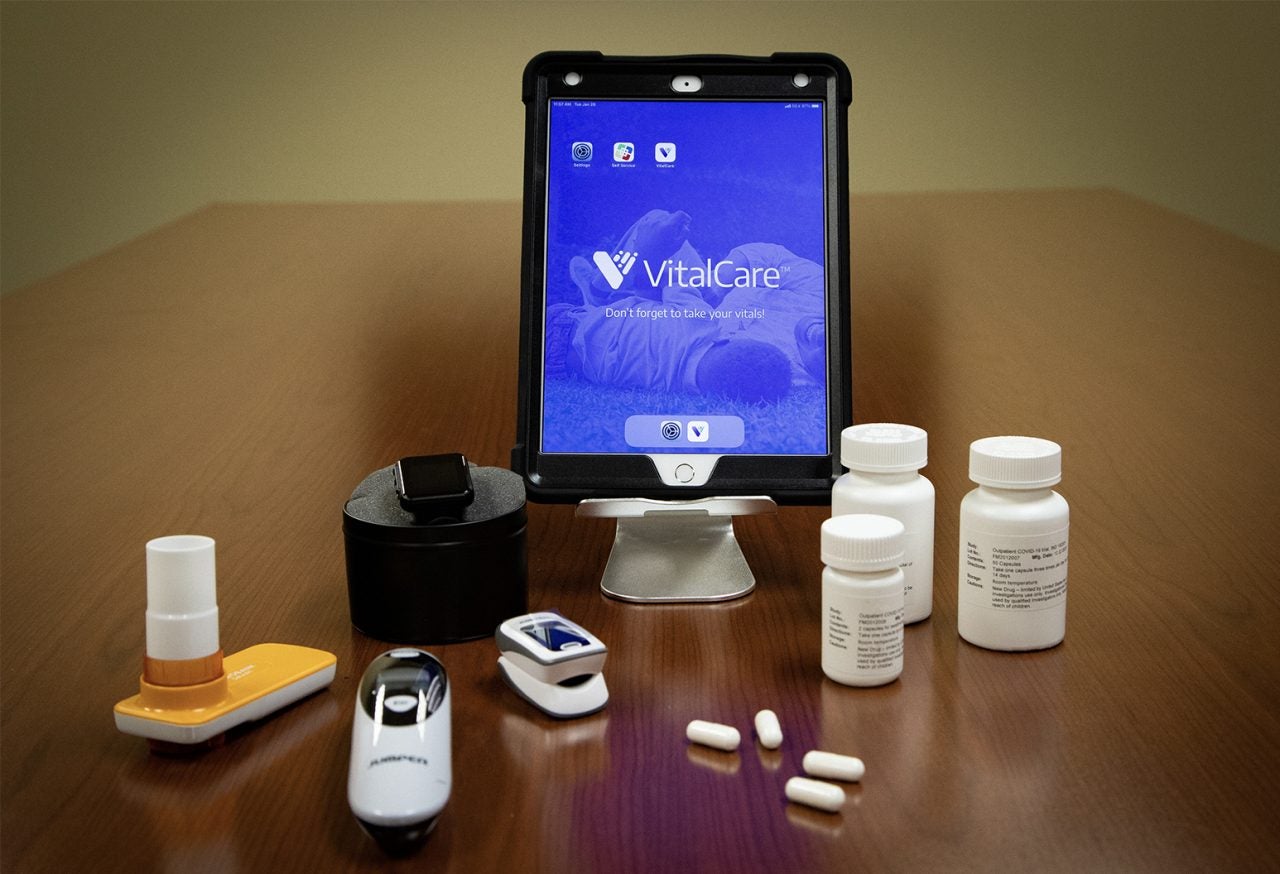
Those enrolled in the study will get trained on how to use a cellular activated Apple iPad from their home, along with a Bluetooth-enabled scale, thermometer, fitness tracker, spirometer, thermometer and pulse oximeter.
The trial will analyse the safety and efficacy of famotidine in treating mild to moderate Covid-19.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn the trial, the patient will be prescribed oral famotidine 240mg or placebo to be taken daily for a maximum of 14 days.
This digital trial is intended to keep the participants completely out of the hospital throughout the treatment course.
CSHL assistant professor, Feinstein Institutes adjunct professor Tobias Janowitz said: “From the comfort of these patients’ own homes, we are taking the traditional clinical trial completely digital to study the efficacy and safety of a potential Covid-19 therapy.
“From assessment, to enrollment and daily data collection, we hope this study’s model will be an example for future clinical trials and will provide high quality data as we assess candidate treatments, like famotidine, to curb this disease.”





