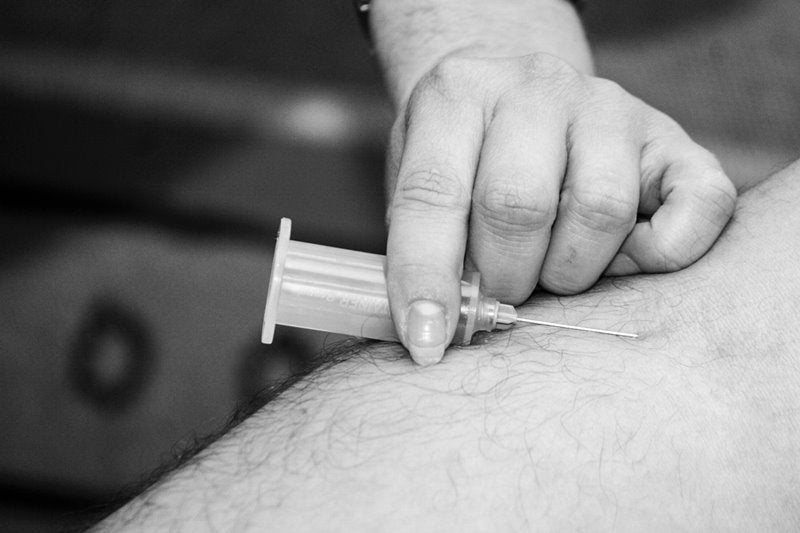
Orphan Technologies has commenced dosing patients in a Phase I/II CBS-HCY-01 clinical trial of OT-58 for the treatment of classical homocystinuria, a rare metabolic disorder.
The disease is characterised by elevated amino acid homocysteine levels that lead to debilitating effects such as severe cardiovascular, skeletal, neurologic and ophthalmologic complications.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
OT-58 is a recombinant enzyme therapy developed to minimise plasma and tissue homocysteine levels.
The double-blind, randomised, placebo-controlled CBS-HCY-01 trial is designed to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and clinical effects of subcutaneous OT-58.
Up to 20 patients suffering from cystathionine beta-synthase deficient homocystinuria will be enrolled in the trail.
The company is also conducting a natural history study to determine progression of disease in homocystinuria patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOrphan Technologies CEO Frank Glavin said: “We believe that OT-58 may be a dramatic advance for these patients and we look forward to the results of this new study.
“In parallel, we are conducting a comprehensive and prospective natural history study of patients with classical homocystinuria as part of our exhaustive approach to understanding and treating this underdiagnosed and underserved disease.”
Primary endpoint of the Phase I part of the CBS-HCY-01 trial is safety, measured as incidence of treatment-emergent adverse events.
The study will also evaluate pharmacokinetic and pharmacodynamic parameters as secondary outcome measures, including changes in total plasma homocysteine and cognitive function.
Participants will also receive eye assessments to check ocular health and dual-energy x-ray absorptiometry (DEXA) to measure bone mineral density. Patient-Reported Outcomes Measurement Information System (PROMIS) will be used to measure the wellbeing of the patient.
The Phase I/II trial was initiated in December last year and is expected to be completed in October.





