Palatin Technologies has started dosing patients in its Phase l clinical trial to assess the safety and tolerability of PL-8177 for the treatment of ulcerative colitis and other inflammatory bowel diseases.
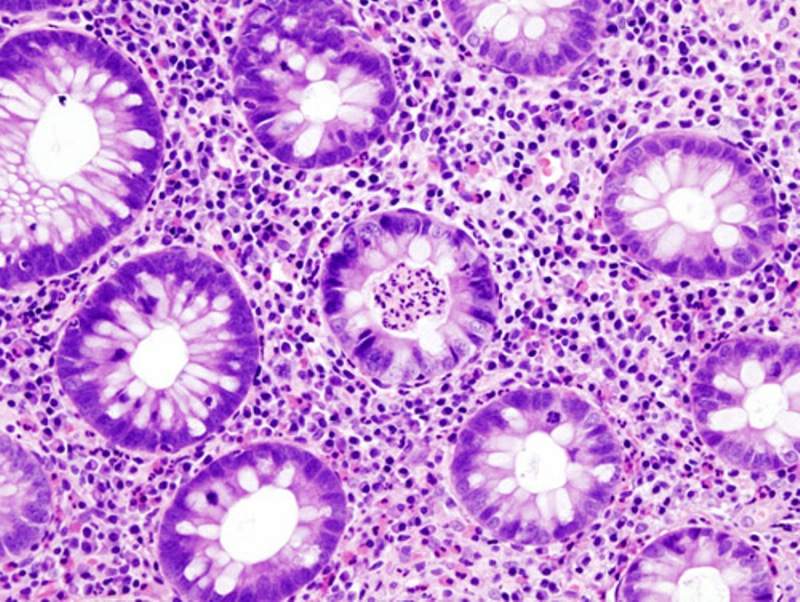
Ulcerative colitis is a chronic disease of the large intestine, with inflammation and ulcerations that can cause symptoms such as significant abdominal pains, persistent diarrhoea and appetite loss.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The first-in-human clinical trial of PL-8177 is a randomised, double-blind, placebo-controlled, single and multiple ascending dose study.
During the trial, PL-8177 will be administered through subcutaneous injection.
The trial aims to enrol up to 52 healthy volunteers and top-line data is expected by the third quarter of this year.
Palatin Technologies chief financial officer and chief operating officer Stephen Wills said: “This is a significant milestone for Palatin, as this is our second melanocortin peptide to enter clinical development.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Beyond bremelanotide, for which we expect to file an NDA with the FDA for female sexual dysfunction later this quarter, we continue to expand our pipeline of novel peptide therapeutics targeting the melanocortin system.
“We are excited about the potential of our melanocortin receptor 1 (MC1r) peptides, which have the potential to treat a wide variety of inflammatory diseases. We currently anticipate additional compounds entering clinical development later this year.”
Palatin’s PL-8177 is a synthetic cyclic heptapeptide that has shown efficacy in animal inflammatory bowel disease models.
The formulation is also a potent agonist at human MC1r, with sub-nanomolar affinity binding and EC50 functional values.





