Pasithea Therapeutics has started enrolling patients in a Phase I trial investigating its candidate for the treatment of neurofibromatosis type 1 (NF1).
The US biotech has activated four clinical sites in the country, with three more to open soon in Eastern Europe.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Pasithea is assessing its macrocyclic mitogen-activated protein kinase (MEK) inhibitor in the open-label Phase I study. The candidate, called PAS-004, will be administered in patients with mitogen-activated protein kinase (MAPK) pathway-driven advanced solid tumours who have a mutation in the RAS, NF1, or RAF gene. Patients who have not responded to BRAF/MEK inhibition will also be eligible for the trial.
The trial will assess the feasibility and anticancer efficacy of the drug and indicate a preliminary dose for a Phase II trial. Pasithea stated it will begin a Phase II trial in paediatric and adult patients with NF1 once safety and pharmacokinetics are established.
Novotech has been appointed as the clinical research organisation (CRO) for the trial. NEXT Oncology will provide US investigators.
Pasithea states that its candidate is the first macrocyclic MEK inhibitor to enter human clinical trials. PAS-004 inhibits both MEK 1 and MEK2 –which when abnormally activated can cause the formation and progression of tumours and fibrosis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThere are a range of MEK inhibitors already approved by the FDA for different types of cancers. This includes AstraZeneca and MSD’s Koselugo (selumetinib), approved in April 2020 for paediatric patients with NF1. The drug generated sales of $208m in 2022, though it has yet to gain expanded adult approval.
Pasithea says the macrocyclic nature of its candidate overcomes the limitations of currently marketed MEK inhibitors. The company reckons the drug has a longer half-life and a stronger efficacy.
As per an orphan drug designation awarded to PAS-004, Pasithea could be in line for seven years of market exclusivity if the therapy is approved.
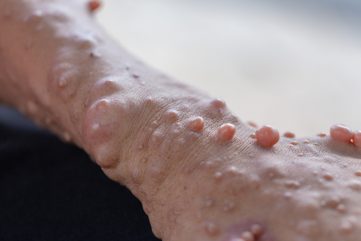
NF1 is a genetic condition that causes changes in skin pigmentation and the growth of tumours along nerves. NF1 is the most common form of neurofibromatosis.
Pasithea’s CEO Dr Tiago Reis Marques said: “We are ready to screen and enrol subjects in the coming month and look forward to gaining insight into the safety, tolerability and initial efficacy of PAS-004.”





