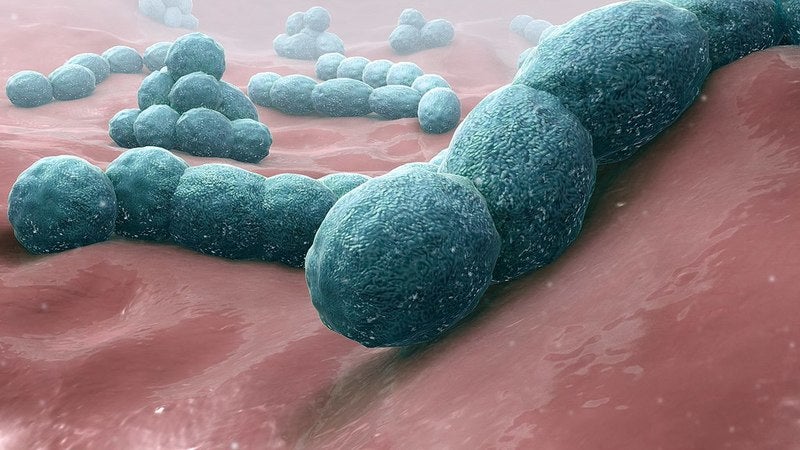
Pfizer has initiated three Phase III clinical trials to evaluate its 20-valent pneumococcal conjugate vaccine (20vPnC) candidate PF-06482077 to prevent invasive disease and pneumonia due to Streptococcus pneumonia in adults.
The vaccine candidate includes a total of 20 serotypes of the bacteria, with seven new ones added to the prior 13 serotypes contained in Prevnar 13.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
All of the new serotypes are global causes of invasive pneumococcal disease, while six are linked to high case-fatality rates. Four of the seven serotypes are also associated with antibiotic resistance and/or meningitis.
The company added that all 20 types in PF-06482077 cause the majority of currently circulating pneumococcal disease in adults.
Pfizer senior vice-president and vaccine R&D head Kathrin Jansen said: “Pneumococcal disease burden remains a large unmet medical need in all age groups with changes in pneumococcal serotype prevalence observed globally, in part driven by antibiotic resistance.
“We carefully monitored and evaluated the global pneumococcal epidemiology over time and selected the additional serotypes in the 20vPnC vaccine candidate with the intent, assuming successful development, to broaden global protection against pneumococcal disease beyond that afforded by existing pneumococcal conjugate vaccines.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe pivotal Phase III development programme will enrol more than 6,000 vaccine-naïve adults, as well as those who previously had pneumococcal vaccination.
The first trial is expected to be completed next year and will assess the safety and immunogenicity of the 20vPnC vaccine candidate in approximately 3,880 vaccine-naïve adults.
The second trial will recruit around 875 subjects aged 65 years or older with prior pneumococcal vaccination. Pfizer expects to complete this trial by December this year.
Three lots of the 20vPnC vaccine candidate will be evaluated in about 1,610 vaccine-naïve adults aged 18-49 years in the third trial, which is also set to be completed by December.





