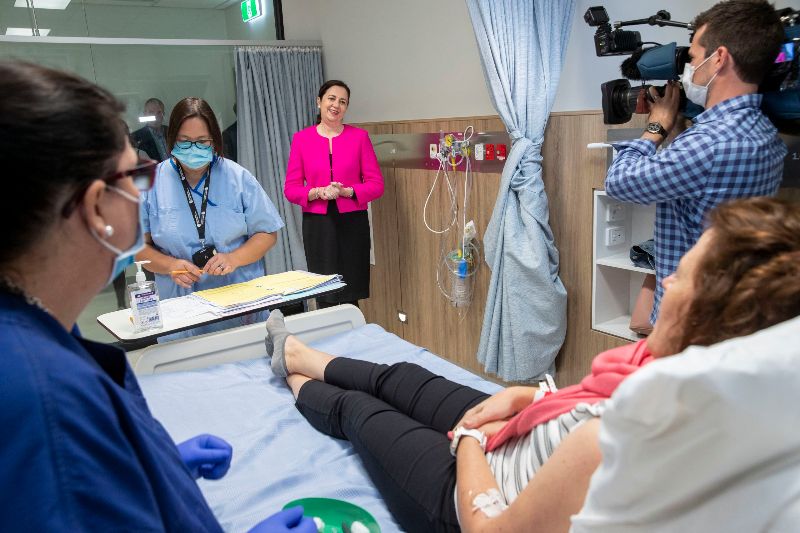
The University of Queensland in Australia has started dosing healthy adult participants in a Phase I clinical trial of its Covid-19 vaccine candidate.
The first dose of the potential vaccine was given at Nucleus Network’s Brisbane clinic. The trial is intended to assess the safety of the candidate, along with its ability to induce immune response in healthy volunteers.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
University of Queensland vaccine project co-leader professor Paul Young said: “The green light to move into this first phase of human trials follows extensive pre-clinical testing that started when we first selected our lead vaccine candidate on 14 February.
“This testing showed that the vaccine was effective in inducing antibodies that were able to neutralise the virus. Further studies have shown that the vaccine was safe to give to people.”
In January, the Coalition for Epidemic Preparedness Innovations (CEPI) asked the university to create a rapid response vaccine against Covid-19, with an initial investment of up to A$4.5m ($3.1m).
During the Phase I trial, approximately 120 participants aged 18 to 55 will be enrolled. Some participants will be administered with a placebo.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPreliminary data is expected to be available in around three months. If positive results are obtained, university researchers intend to perform a larger trial with a broader population.
The clinical batch of the vaccine candidate for use in the trial was produced in alliance with researchers at CSIRO’s advanced biologics production facility in Melbourne. Biotech company CSL, as well as Patheon and Cytiva, provided technical assistance.
The aim is for CSL to rapidly accelerate the manufacturing of millions of doses and advance the programme into later stage clinical testing, regulatory approval, large-scale production, and distribution.
The Queensland Government provided A$10m ($6.9m) in funding for the vaccine project, while the Federal Government offered A$5m ($3.4m). More than A$10m was obtained from philanthropic and other donors.
Earlier this month, clinical trials of a Covid-19 vaccine developed in Australia have commenced at the Royal Adelaide Hospital’s PARC Clinical Research facility.





