Ra Pharmaceuticals has reported positive data from the Phase lb trial examining the pharmacokinetic (PK) of zilucoplan (RA101495 SC) to treat patients with renal impairment.
The trial investigated zilucoplan in severe renal impairment patients as a lead-in to studying the drug in complement-mediated renal disorders.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
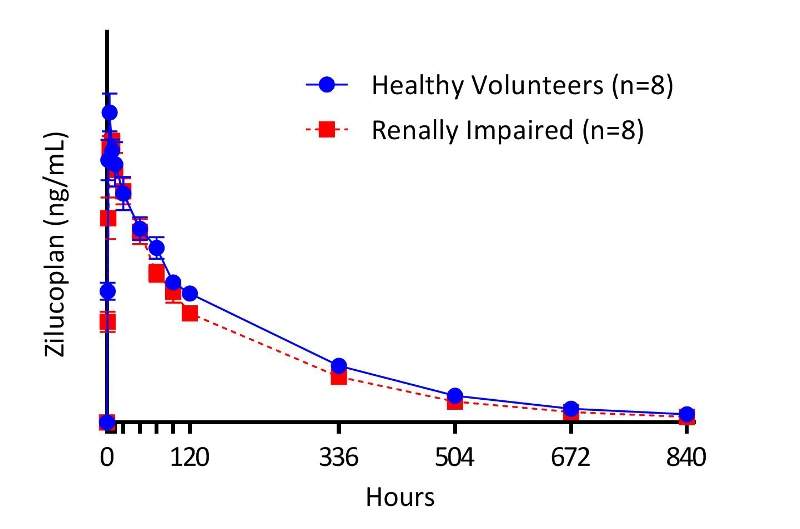
The multi-centre, open-label trial included 16 participants, comprising eight patients with severe renal impairment and eight healthy control subjects with normal renal function.
During the study, each patient was administered with a single, subcutaneous dose of 0.3mg/kg of zilucoplan.
Results revealed that the PK profile of zilucoplan was consistent across both study groups, with exposures similar in renally impaired patients and healthy subjects.
No adverse events were reported during the trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOn the whole, the data suggested that zilucoplan can be used in clinical studies of patients with renal impairment with the same dose as used in the Phase lb trial.
Ra Pharma CEO Doug Treco said: “These results allow Ra Pharma to expand into complement-mediated renal disorders, adding to a now broad portfolio of clinical programmes that includes our Phase lll-ready programme in PNH and an ongoing Phase ll programme in generalised myasthenia gravis (gMG).
“In each of these indications, we believe zilucoplan, as a once-daily, subcutaneously self-administered therapy, offers the potential to address broader patient populations with complement-mediated diseases by offering greater access and convenience.
“We look forward to a number of important milestones ahead, including top-line data in gMG around year-end 2018.”
In addition, Ra Pharma recently completed discussions with regulators, including the US Food and Drug Administration, to begin a Phase lll study of zilucoplan for the treatment of paroxysmal nocturnal haemoglobinuria (PNH).
As a result of these discussions, the company is expected to start the global, pivotal, single-arm Phase lll trial during the first half of next year.





