Biopharmaceutical company Regulus Therapeutics has completed enrolment of the first cohort of subjects in its Phase Ib clinical study of RGLS4326 in autosomal dominant polycystic kidney disease (ADPKD) patients.
RGLS4326 is a novel oligonucleotide made to stop miR-17 and specifically target the kidney.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Last July, the US Food and Drug Administration (FDA) granted orphan drug designation to RGLS4326.
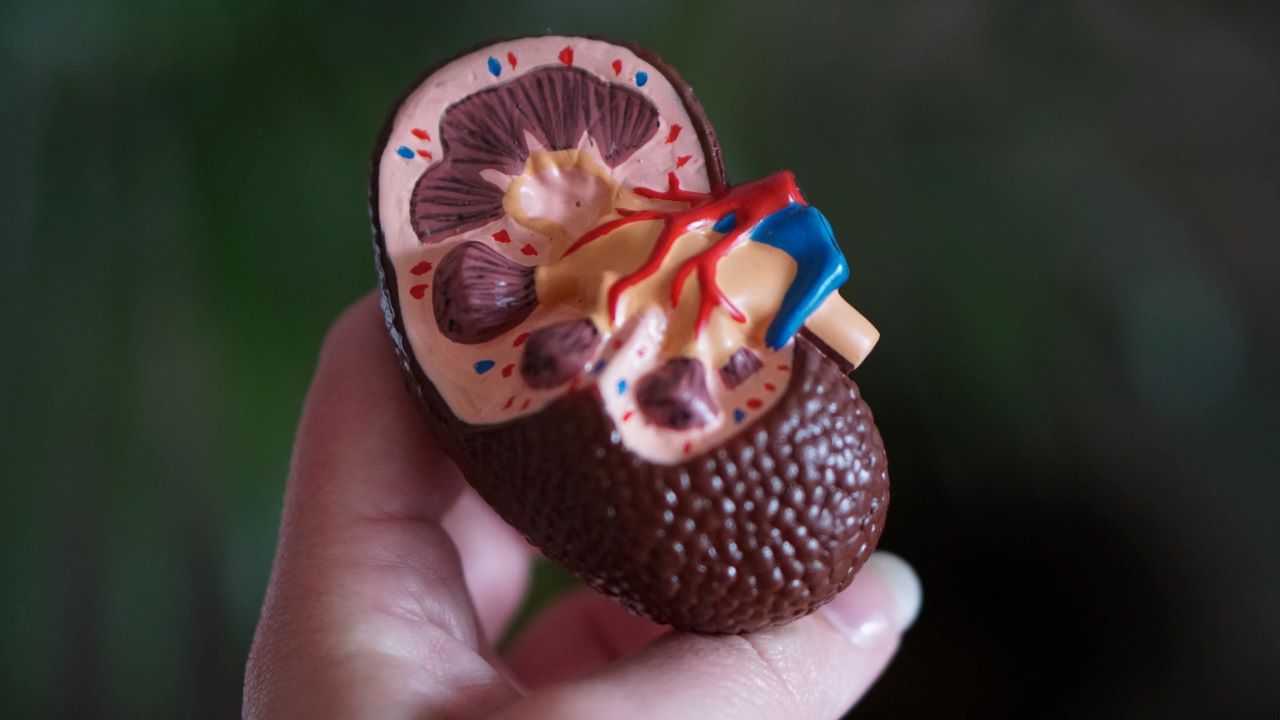
Caused by PKD1 or PKD2 gene mutations, ADPKD is the most common human monogenic disorders and a major cause of end-stage renal disease.
It is characterised by multiple fluid-filled cyst development, mainly in the kidneys and the liver.
The adaptive design, open-label, multiple-dose study will analyse the safety, pharmacokinetics and changes in levels of polycystin 1 (PC1) and polycystin 2 (PC2) in ADPKD patients receiving RGLS4326 every other week for a total of four doses.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt has up to three cohorts and the dose levels for the first and second cohort are RGLS4326 1mg/kg and 0.3mg/kg, respectively.
The third and final cohort will be dosed at a level to be determined based on the results of the first two cohorts.
On concluding enrolment in the first cohort and based on the review of the available interim safety data, the first patient of the second cohort was given the first dose of RGLS4326.
Regulus Therapeutics CEO Jay Hagan said: “We are pleased to have reached this important step in our ongoing RGLS4326 programme for the treatment of ADPKD.
“To date, no serious adverse events have been reported and results from the first cohort are expected to be available early in the second quarter of this year.”
In December 2017, Regulus Therapeutics started a Phase I trial of RGLS4326 for the treatment of ADPKD.





