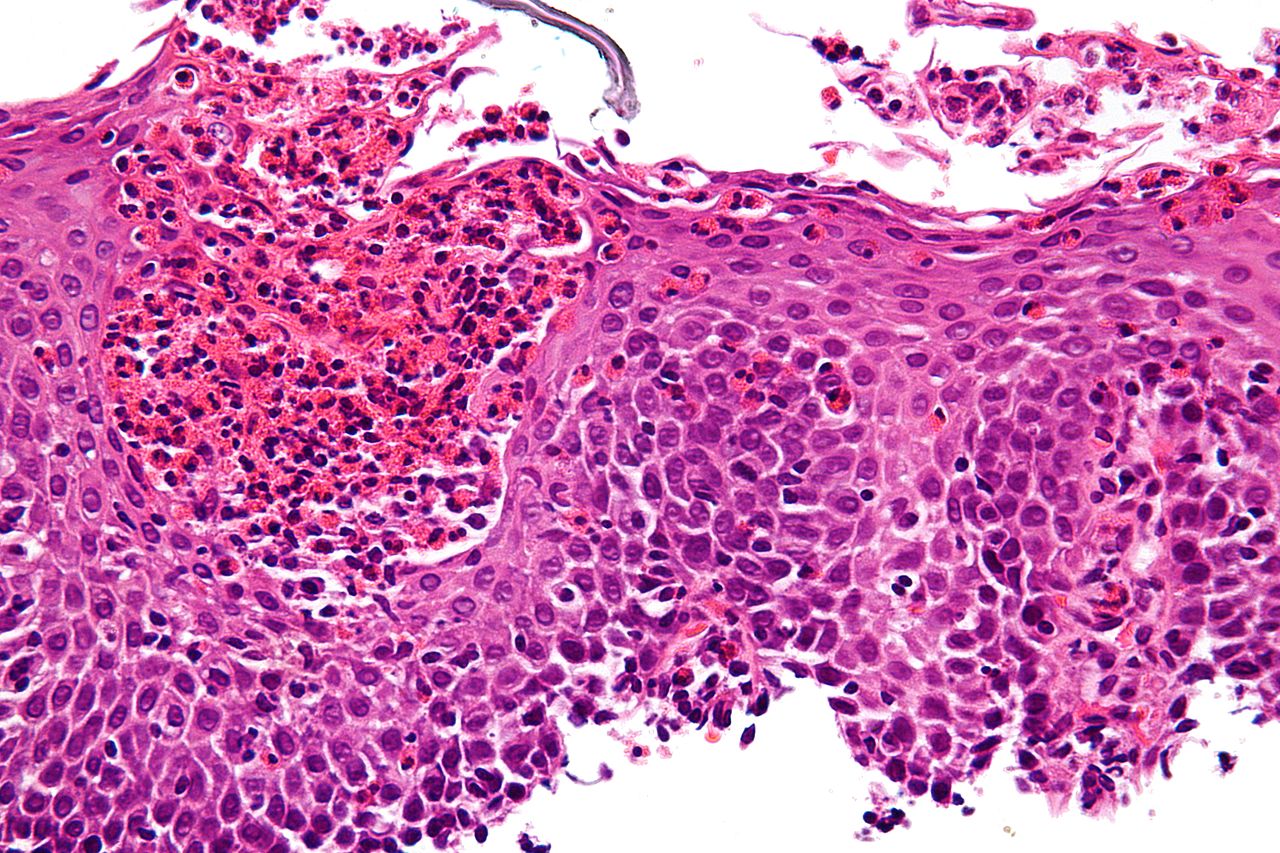
Sanofi and Regeneron Pharmaceuticals have reported additional positive results from Part A of a Phase III trial evaluating Dupixent (dupilumab) in patients with eosinophilic esophagitis (EoE).
Data showed additional improvements in disease severity and extent at the microscopic level and normalisation of gene expression pattern associated with type 2 inflammation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dupixent is a monoclonal antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13) protein signalling, the main drivers of type 2 inflammation.
EoE is a progressive inflammatory disease that damages the oesophagus. Excessive type 2 inflammation can also result in scarring and narrowing of the oesophagus.
Part A of the pivotal Phase III, randomised, double-blind, placebo-controlled trial evaluated the efficacy and safety of Dupixent in EoE patients aged 12 or above.
It enrolled 81 patients, who were given a weekly dose of 300mg Dupixent or placebo for 24 weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial’s co-primary endpoints were the change from baseline in the Dysphagia Symptom Questionnaire (DSQ), a patient-reported measure of difficulty swallowing and patients achieving peak oesophagal intraepithelial eosinophil count of less than or equal to 6eos/hpf at 24 weeks.
The key secondary endpoint of the trial analysed histopathologic measures of the severity and extent of tissue scarring in the oesophagus and the proportion of patients achieving peak oesophagal intraepithelial eosinophil count of above 15eos/hpf at 24 weeks.
Trial principal investigator and University of North Carolina, School of Medicine Gastroenterology and Hepatology Professor Evan Dellon said: “The results from this trial show dupilumab significantly improved both patients’ ability to swallow, as well as structural abnormalities in the oesophagus, by targeting type 2 inflammation to help reverse tissue damage and scarring that usually worsens over time.”
Data showed that patients treated with Dupixent reported rapid improvement in ability and comfort of swallowing as early as four weeks and continued to improve through 24 weeks.
About 64% of patients given Dupixent achieved less than 15 eos/hpf versus 8% for placebo at 24 weeks.
Peak oesophagal eosinophil count was lowered by 71% with Dupixent versus 3% with placebo.
In April, the companies reported positive results from the Phase III trial of Dupixent in children aged six to 11 with severe atopic dermatitis.





