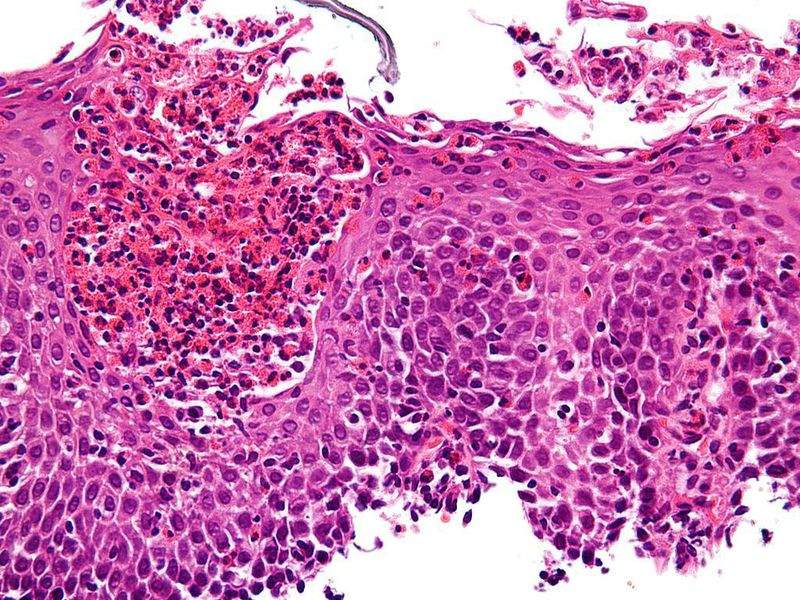
Sanofi and Regeneron have reported positive results from a Phase II clinical trial of dupilumab for the treatment of adults with active moderate to severe eosinophilic oesophagitis.
Dupilumab is a human monoclonal antibody being developed for simultaneous inhibition of overactive signalling of IL-4 and IL-13 cytokines that are reportedly involved in type 2 inflammation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Results indicated that subjects administered weekly with dupilumab experienced a significant improvement in the ability to swallow at week ten, when compared with placebo.
In a total of 47 patients, the randomised, 12-week Phase II trial evaluated 300mg dupilumab after a 600mg loading dose.
The trial’s primary endpoint was change in swallowing difficulty from baseline to week ten measured as Straumann Dysphagia Instrument (SDI) score.
Northwestern University Feinberg School of Medicine professor Ikuo Hirano said: “Currently, there are no US Food and Drug Administration (FDA) approved therapies for eosinophilic oesophagitis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“These positive Phase II results support further clinical development of dupilumab for patients with eosinophilic oesophagitis.”
Dupilumab is reported to have achieved the Phase II trial secondary endpoints, including decreased mean change in the Eosinophilic Oesophagitis Endoscopic Reference Score (EoE-EREFS), overall peak intraepithelial eosinophil count, and composite measures of symptoms and quality of life.
The FDA has granted orphan drug designation to dupilumab for the treatment of eosinophilic oesophagitis.
Additionally, the drug candidate is being studied by the firms in separate Phase III trials for paediatric atopic dermatitis, uncontrolled persistent asthma and nasal polyps.





