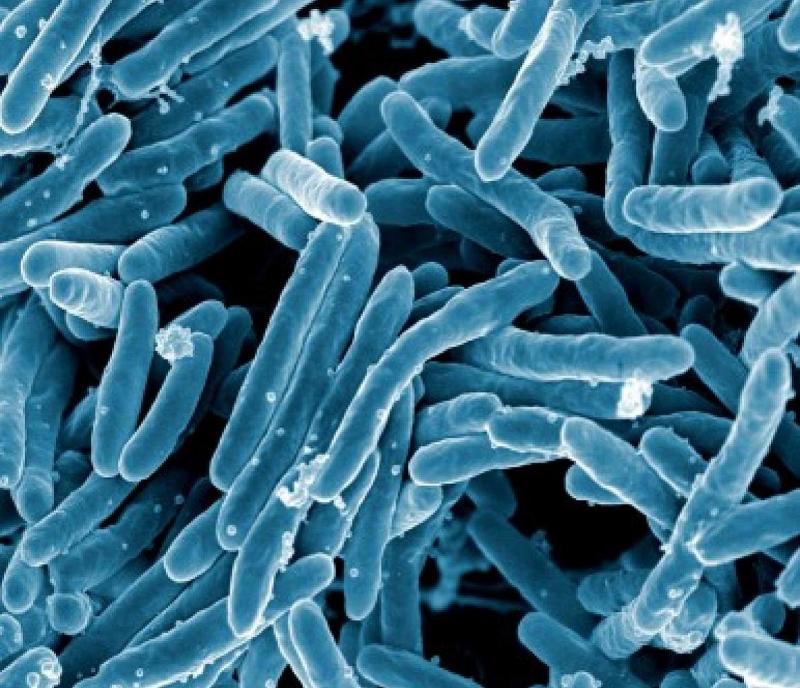
Scientists from the National Institute of Allergy and Infectious Diseases (NIAID) have started vaccinations in a Phase I clinical trial to evaluate ID93 for the treatment of tuberculosis (TB).
ID93 is a freeze-dried, temperature-stable TB vaccine candidate developed by scientists at the US Infectious Disease Research Institute (IDRI).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase I trial is designed to investigate the safety, tolerability, and immunogenicity of single-vial formulation ID93 and GLA-SE vaccine.
It is being conducted at the Saint Louis University School of Medicine Center for Vaccine Development and is expected to include around 48 healthy adult subjects aged 18 to 55.
Patients will receive two vaccinations within a period of 56 days. Half will receive the single-vial formulation of ID93 and GLA-SE, while the remaining subjects will be treated with the previously tested two-vial presentation of powdered ID93 and liquid GLA-SE.
All subjects will be observed for any possible reactions to the vaccine and will provide blood samples at specified time points over a period of around seven months.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe samples will be examined by the trial’s investigators to find out whether the subjects develop an immune response.
A safety monitoring committee comprising an independent group of experts will review data during the trial to ensure the safety of the subjects.
Saint Louis University School of Medicine Division of Infectious Diseases, Allergy and Immunology director Daniel Hoft is the principal investigator of the trial.
The trial is being supported by National Institute of Allergy and Infectious Diseases (NIAID) as part of a contract with IDRI.
The NIAID contract principal investigator and IDRI Formulations vice-president Christopher Fox said: “To our knowledge, the freeze-dried formulation of ID93 + GLA-SE represents the first time a thermostable vaccine candidate containing a modern immune-boosting substance has reached clinical testing.
“Implementing technologies designed for low-resource settings early in product development could help accelerate vaccine rollout in hard-to-reach areas.”
The study is expected to be completed in November next year.





