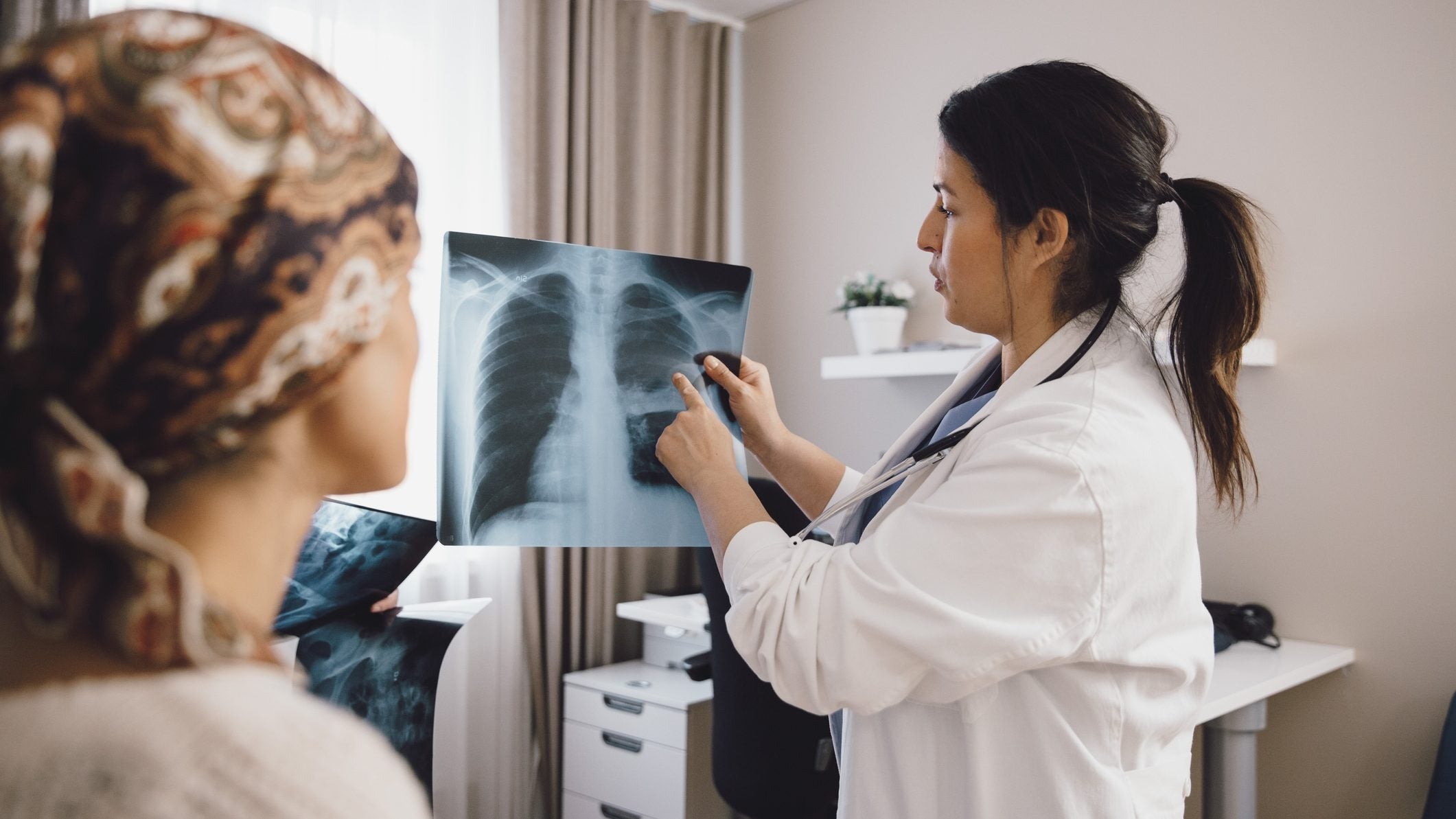
Seagen has reported positive topline data from the Phase III HER2CLIMB-02 clinical trial investigating Tukysa (tucatinib) in combination with the antibody-drug conjugate Kadcyla (ado-trastuzumab emtansine) in patients with previously treated HER2-positive metastatic breast cancer.
In August 2023, Seagen announced that the primary endpoint of the trial of progression-free survival (PFS) has been met, however, the company did not release any data.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Patients who received Tukysa and Kadcyla achieved a PFS of 9.5 months compared to 7.4 months in the placebo and Kadcyla alone cohorts. Patients with brain metastasis had a PFS of 7.8 months compared to 5.7 months in patients in the placebo arm.
Overall survival data, which is the secondary endpoint, has not yet matured, as per a 6 December press release.
The multicentre, randomised, double-blind trial enrolled 565 patients who had had prior treatment with taxane and trastuzumab.
In the announcement accompanying the data, lead study author Sara Hurvitz said: “Combining HER2-directed therapies can improve outcomes for people with locally advanced or metastatic HER2-positive breast cancer. Notably, the HER2CLIMB-02 trial is the second randomised study including patients with brain metastasis demonstrating that Tukysa delays disease progression in this population.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTukysa is a tyrosine kinase inhibitor of HER2 and first gained approval by the US Food and Drug Administration (FDA) in April 2020 to treat adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting. Merck (MSD) has exclusive rights to commercialise Tukysa in regions outside of the US, Canada, and Europe.
Last year, Tukysa generated $353m in sales, as per Seagen’s 2022 financial results. According to GlobalData’s Pharma Intelligence Center, Tukysa is forecasted to generate $1.6bn in sales in 2029.
GlobalData is the parent company of Clinical Trials Arena.
Seagen is also investigating Tukysa as a treatment for colorectal cancer in the Phase III MOUNTAINEER-03 trial (NCT05253651). The trial plans to enrol 400 patients and has a primary completion date of July 2025. The open-label, randomised study evaluates Tukysa with trastuzumab and MFOLFOX6 versus standard-of-care treatment in first-line HER2+ metastatic colorectal cancer. The primary endpoint of the study is PFS.





