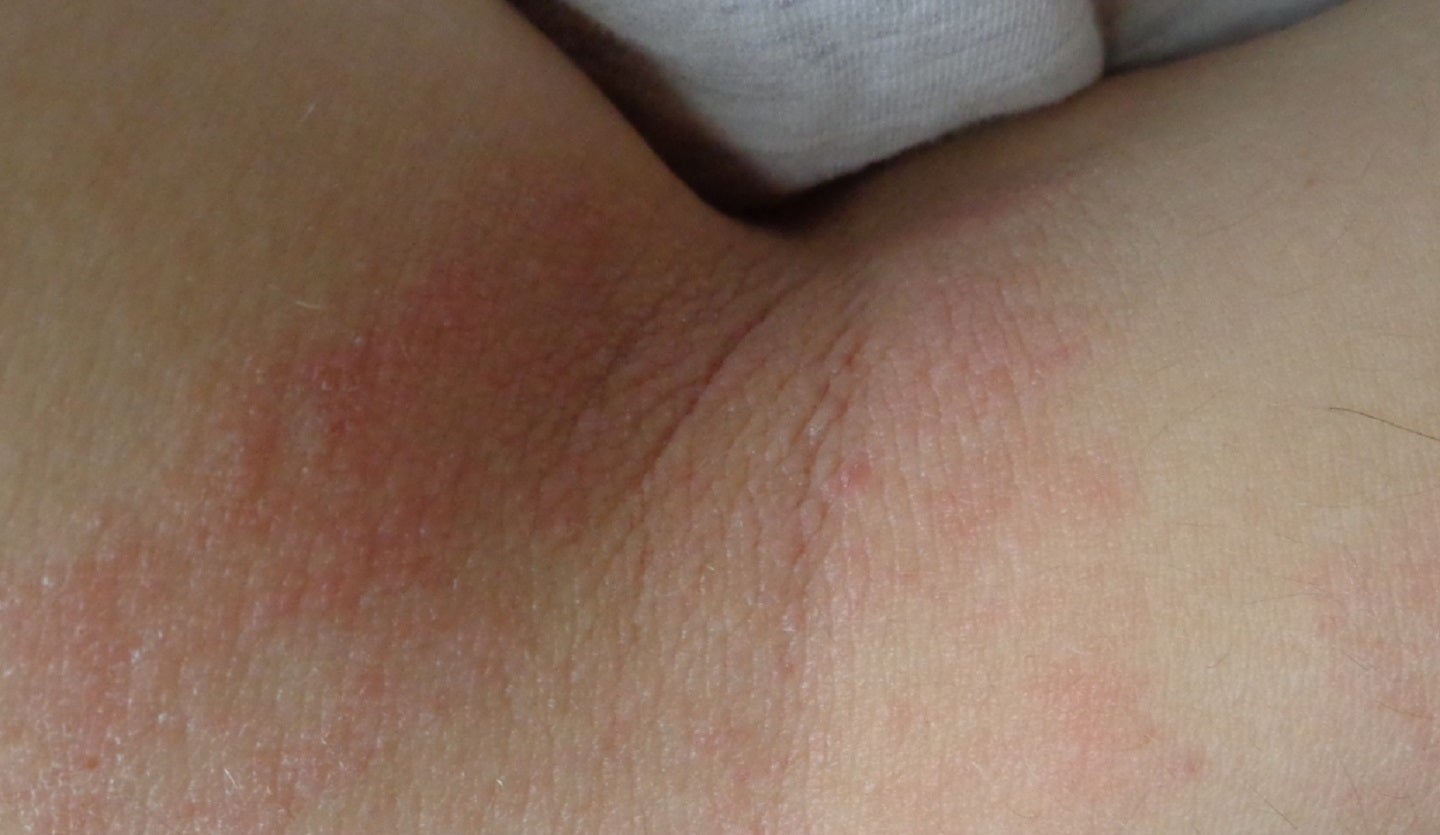
selectION has reported initial safety results from the single ascending dose (SAD) stage of its ongoing Phase Ib trial of si-544 for the treatment of atopic dermatitis.
si-544 is a selectivity-optimised peptide that blocks the Kv1.3 ion channel and helps in the activation and proliferation of TEM cells.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The placebo-controlled, double-blind, multi-centre study is designed to assess the tolerability, safety, and efficacy signals of the drug candidate in patients with mild to severe atopic dermatitis.
No safety signals or dose limiting toxicities have been observed during the study’s first stage.
selectION chief scientific officer and co-founder Andreas Klostermann said: “The ion channel Kv1.3 controls the activation and proliferation of auto-reactive effector memory T-cells and has been regarded a key target in T-cell autoimmunity for decades.
“So far, it has not been possible to block this ion channel with sufficient selectivity.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The initial analysis of safety and tolerability data from 20 patients confirms that si-544 can be safely administered at dose levels sufficient to achieve virtually full Kv1.3 target engagement.”
Based on the initial results, selectION now progresses the multiple ascending dose (MAD) stage of the Phase Ib trial.
Patients in this study will be treated with si-544 for a period of one month and thereafter monitored for three months.
selectION chairman and CEO Antonius Schuh said: “The data provide initial clinical validation that we can safely and selectively target autoimmune disease associated chronically activated effector memory T-cells.
“We will continue our path to deliver a potent and immune-selective therapy which maintains a patient’s general immunocompetency, and we believe that si-544 has the potential to significantly improve safety and outcomes for patients suffering from a wide range of autoimmune diseases, including atopic dermatitis, psoriasis, psoriatic arthritis, rheumatoid arthritis, multiple sclerosis, and many others.”





