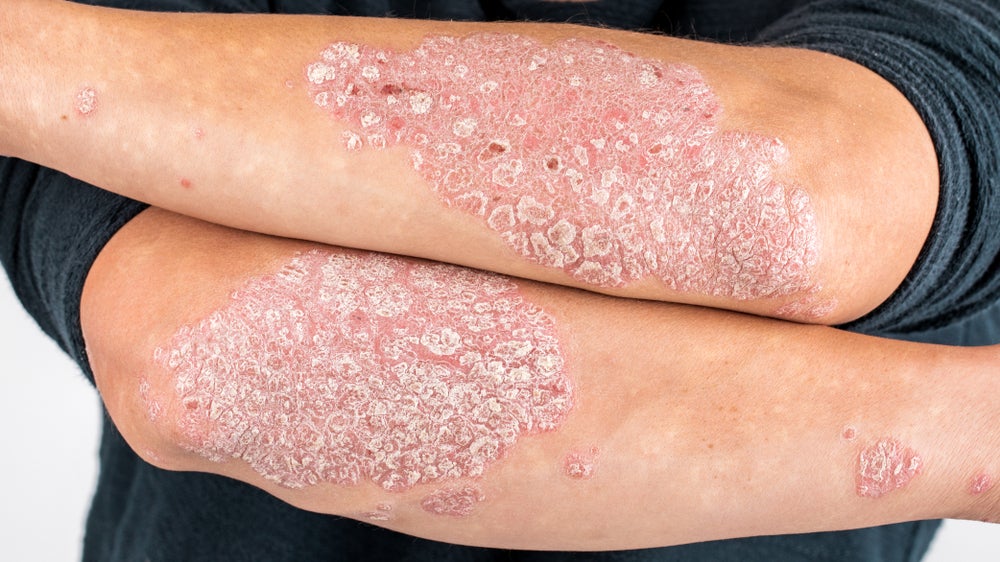
SFA Therapeutics has announced that 92% of patients with plaque psoriasis achieved a Psoriasis Area and Severity Index (PASI) score of greater than 50 in the Phase Ib trial investigating its SFA-002.
The clinical-stage biotech announced extension results from its Phase Ib (NCT05642182) study which is investigating the candidate in mild-to-moderate chronic plaque psoriasis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The ongoing open-label trial is evaluating two different formulations of SFA-002. Participants receive 12 weeks of active therapy and one month of follow-up with an optional treatment extension of 12 weeks.
The primary outcome of the study is safety, with secondary efficacy outcomes including the International Global Assessment (IGA) and PASI scores, according to SFA’s press release on 9 November.
SFA has also announced bridge financing of $4.5m, which was led by North South Ventures. The funds will allow SFA-002 to advance through the ongoing Phase Ib and its second cohort. Final data is expected in H1 2024. The funds will also allow SFA to plan the Phase II trial of the candidate.
SFA-002 Phase Ib first cohort extension results
The analysis of 14 subjects, including six subjects on the three-month extension, showed that 92% of patients achieved a PASI improvement of greater than 50, with 72% showing improvement of greater than PASI 75. Two patients achieved PASI 100, which is 100% clearance.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis is an improvement on the first set of results released in August 2023, which showed 85% of patients achieving a PASI 50 and 71% achieving a PASI 75.
Treatment onset action was observed in some patients at six weeks. No adverse events or toxicities related to treatment were observed during trial or follow-up.
About SFA-002
SFA-002 is the lead asset in the SFA Therapeutics portfolio, serving as a proof-of-concept for SFA’s key mechanism of action aimed at treating a range of autoimmune diseases.
The oral pill acts simultaneously on multiple therapeutic pathways. The candidate has the potential to be disease-modifying and will create a new class of immunomodulators that work by blocking autoimmunity instead of immunosuppression.
According to GlobalData’s Pharmaceutical Intelligence Centre, SFA therapeutics has six pipeline candidates and is currently running two clinical trials, with another trial being planned.
GlobalData is the parent company of Clinical Trials Arena.





