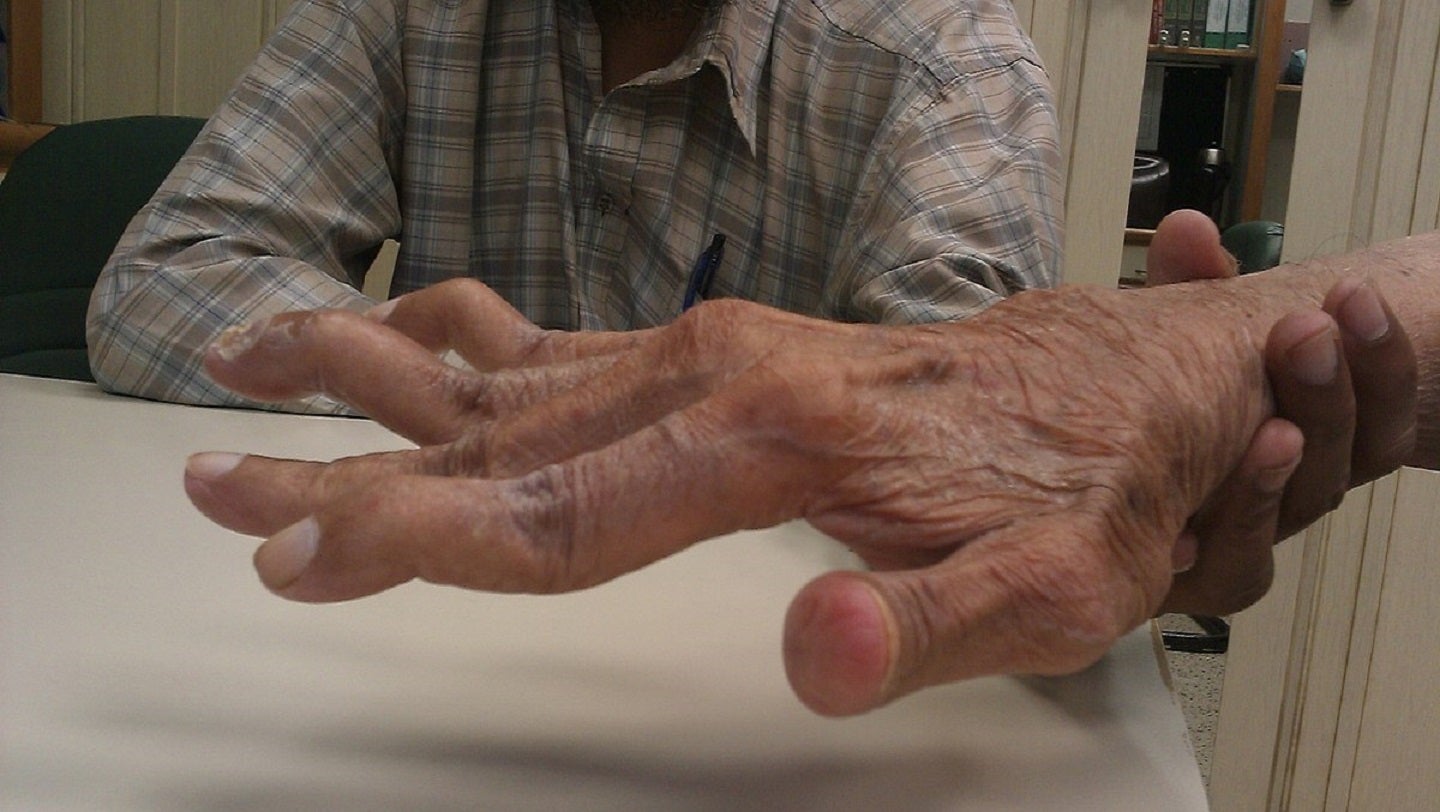
SinoMab BioScience has reported that its Phase III trial of SM03 (Suciraslimab) to treat rheumatoid arthritis (RA) in China has concluded unblinding and preliminary statistical analysis and met the primary endpoint.
Designed to evaluate the safety and efficacy of SM03, the randomised, multi-centre, double-blind, placebo-controlled study has included patients with moderate-to-severe active RA who did not respond well to methotrexate (MTX).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Suciraslimab could suppress disease activity and mitigate symptoms of patients with RA receiving MTX therapy.
Measuring the percentage of participants with ACR 20 response at week 24 is the primary endpoint of the study.
A composite measure, ACR20 is designed for the assessment of RA improvement by the American College of Rheumatology.
SinoMab executive director, chairman and CEO Dr Shui On LEUNG said: “The market for therapeutics for autoimmune diseases is in a stage of rapid growth, in which the demand for RA treatment is high.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“However, there are limitations on the efficacy and safety of currently available drugs for RA treatment. Suciraslimab, the company’s flagship product, is the world’s first CD22- targeted new drug that is expected to be approved for marketing for the treatment of RA.
“The Phase III clinical data readout has successfully reached the primary endpoint and further demonstrated the efficacy of the new drug.”
The company is planning to submit biologics license application to the National Medical Products Administration in the third quarter of this year.





