South Korea-based SK bioscience is set to receive up to $173.4m (KRW200bn) in funding from the Coalition for Epidemic Preparedness Innovations (CEPI) to support the Phase III trial of its Covid-19 vaccine candidate, GBP510.
The funds will also be used for other development work related to the vaccine, including regulatory activities, commercial processes development, buying raw materials and research and development on response to variants.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
SK bioscience is developing the nanoparticle vaccine candidate in partnership with the Institute for Protein Design at the University of Washington (UW) in the US. The researchers aim to develop a vaccine that works at low doses.
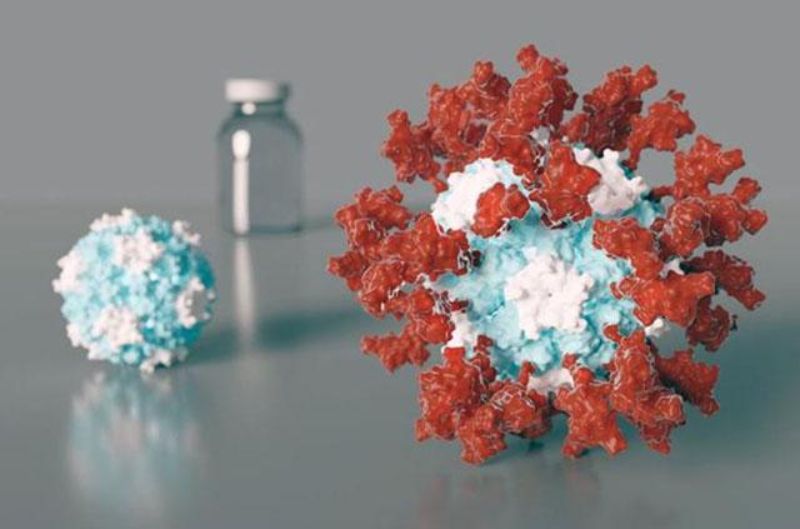
Designed using UW Medicine’s structure-based vaccine design methods, GBP510 builds on a computationally developed self-assembling protein nanoparticle. It has 60 copies of an important region of the coronavirus Spike protein.
SK bioscience intends to file an investigational new drug application with South Korea’s Ministry of Food and Drug Safety and other regulators next month to conduct a multi-national Phase III trial of GBP510.
At the end of last year, a Phase I/II trial of the vaccine candidate was launched and is currently in its second stage. The company said that the trial has so far yielded promising safety and immunogenicity data.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn preclinical studies, low doses of the vaccine candidate were able to generate high virus-neutralising antibody levels, which act on multiple viral Spike protein sites. This indicates potential for improved protection against the Covid-19 virus variants.
Additional preclinical data showed a strong B-cell response, which could allow long-term protection. SK bioscience laboratory studies revealed that the vaccine candidate inhibited viral proliferation.
SK bioscience CEO Jae-Yong Ahn said: “To achieve the goals against the pandemic, we will continue our highest efforts for the successful development and enlarged access to a Covid-19 vaccine, that can also be preventive against variants, by utilising our expanded manufacturing capabilities.”
The company previously received funding from the Bill & Melinda Gates Foundation to support preclinical laboratory studies and from CEPI for preliminary trials, variants research and development of manufacturing process.
The latest CEPI funds take the total investment in SK bioscience to $210.1m.





