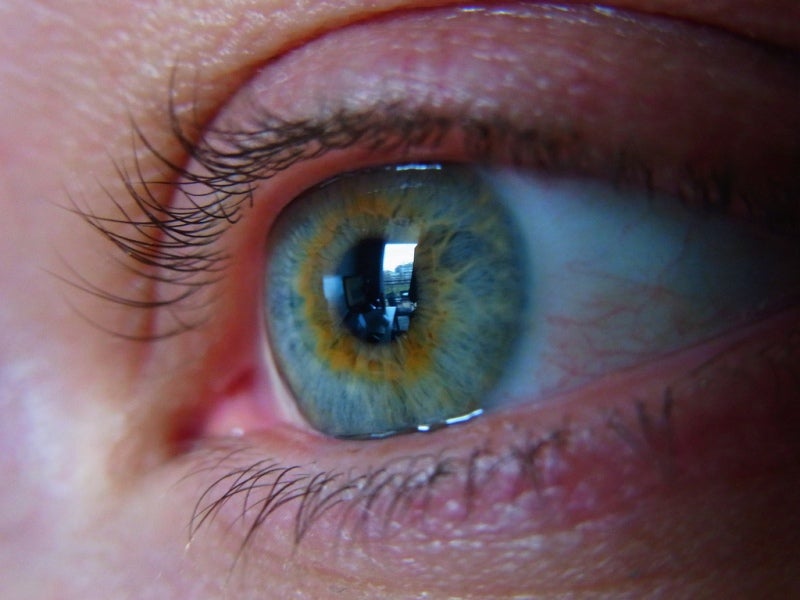
Sylentis’ tivanisiran has failed to meet the primary objectives of the Phase III HELIX trial evaluating the efficacy and safety of the drug in comparison with artificial tears for the treatment of dry eye disease.
The unmet primary objectives of the trial included ocular pain and total corneal staining. Results showed no statistically significant difference between tivanisiran and the comparator.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
However, the trial demonstrated a clinically relevant improvement in the primary objective of total corneal staining.
The trial met its secondary objective of achieving improvement by tivanisiran compared to the comparator in lowering central corneal staining in patients with moderate to severe dry eye disease after one month of treatment.
An improvement was also seen in ocular pain symptoms and sign of corneal staining was observed in the patients with respect to baseline.
Several biomarkers of the disease were also identified as part of the trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataResults showed superior characteristics of tivanisiran over the comparator with a 125% increase in mucin and a 13% decrease in the inflammation marker.
The trial did not report any serious adverse effects related to the use of tivanisiran.
HELIX trial principal investigator José Manuel Benítez del Castillo said: “The need to find a solution to this disease is very high. In Spain alone, one out of five visits to the consultants are motivated by this pathology.
“Symptoms manifested by patients include pain, dry eyes, stinging, burning, a sensation of having a foreign body in the eye and could also include episodes of blurred vision.
“Tivanisiran could be a therapeutic alternative for these patients as it is a drug with a new mechanism of action.”
The HELIX trial included 330 patients with dry eye disease at 39 centres in Spain, Germany, Estonia, Portugal, Slovakia, and Italy.
The study saw tivanisiran compared to artificial tears after its administration once a day for four weeks.





