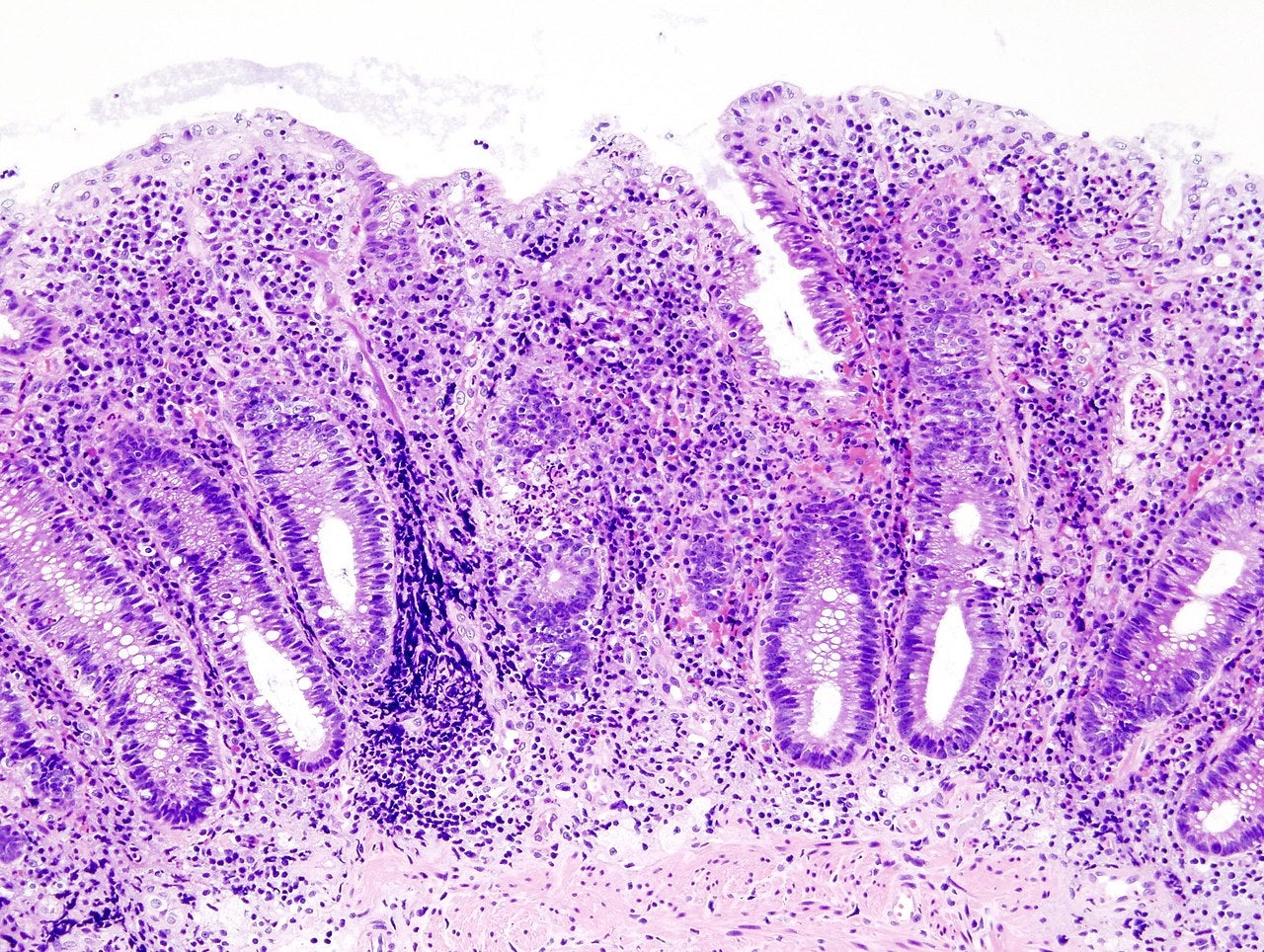
Takeda Pharmaceutical has reported positive interim results from the VISIBLE open-label extension (OLE) trial of a subcutaneous formulation of Entyvio (vedolizumab) in patients with moderately to severely active ulcerative colitis (UC).
Approved in both intravenous (IV) and subcutaneous (SC) formulations, Entyvio is a gut-selective biologic.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Comprising three Phase III studies, the VISIBLE clinical trial programme will involve over 1,000 UC and Crohn’s disease (CD) patients, which includes two randomised, double-blind, placebo-controlled studies.
The ongoing Phase IIIb multinational, multi-centre study will evaluate adults with UC enrolled in the VISIBLE 1 trial and patients with CD in VISIBLE 2 trial.
It will analyse the long-term safety and efficacy of the maintenance treatment with the subcutaneous Entyvios.
Patients who completed the 52-week maintenance period or who achieved clinical response at week 14 after a third IV infusion of Entyvio at week six were administered Entyvio SC 108mg every two weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to the interim results from the study, UC patients continued to demonstrate clinical benefit from the therapy, with rates of clinical remission and corticosteroid-free clinical remission maintained up to week 108.
Long-term safety findings were also consistent with the known safety profile of Entyvio.
Around 69% of UC patients showed adverse events but were consistent with the drug’s known safety profile.
Takeda Gastroenterology Therapeutic Area Unit head Asit Parikh said: “These latest safety and effectiveness data for vedolizumab SC provide additional support/data that the benefits received from subcutaneous vedolizumab are sustained during long-term maintenance therapy.
“Entyvio SC was approved by the European Commission in May 2020, offering greater choice on mode of administration in line with the diverse medical needs and preferences of patients, plus the option of home-administration outside of the medical setting.”
In July 2018, Takeda reported positive top-line results from the VISIBLE 1 trial after meeting its primary endpoint.





