The US Army Medical Research and Development Command (USAMRDC) Office of Human and Animal Research Oversight (OHRO) has granted clearance to Immuron to initiate a Phase II clinical trial of Travelan.
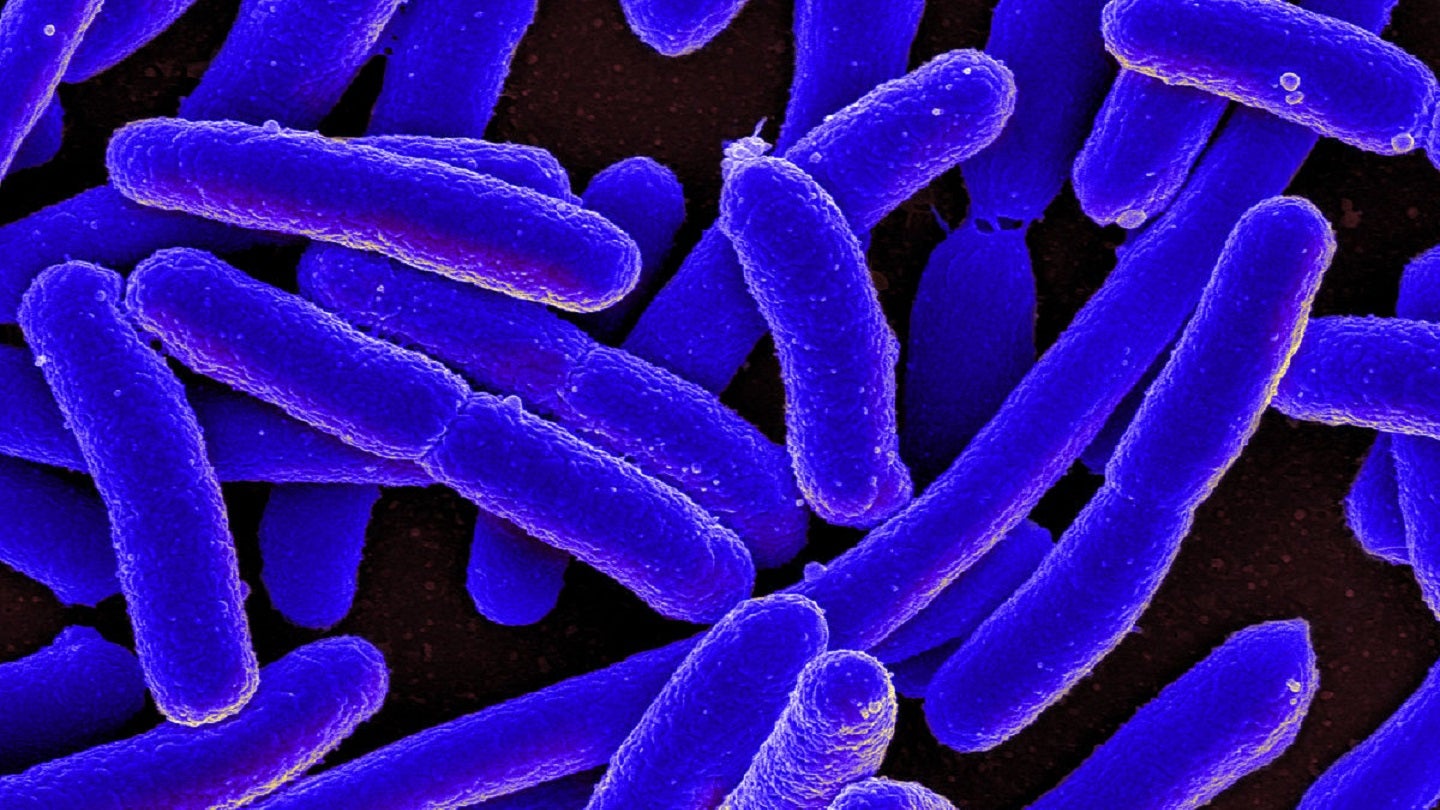
The study will evaluate Travelan’s efficacy in preventing infectious diarrhoea caused by enterotoxigenic Escherichia coli (ETEC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will also demonstrate the protective efficacy and safety of Travelan against a placebo in a controlled human infection model.
In October last year, Immuron executed a master service agreement with Pharmaron CPC for the trial of Travelan.
As part of the planned clinical trial in the US, Pharmaron CPC will conduct the study at its clinical research facility inpatient unit in Baltimore, Maryland.
The study has enrolled up to 60 healthy volunteers including males or non-nursing, non-pregnant females aged 18-50 years.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOut of the total patients, 30 are included in the first cohort and are expected to be enrolled and receive the Travelan dosage by the end of July.
The remaining participants are anticipated to be recruited in October.
Prevention and/or reduction of moderate to severe diarrhoea is the primary efficacy outcome of the study.
A randomised study of Travelan in up to 868 participants is also carried out by the US Department of Defense Uniformed Services University.
The company is planning to initiate two planned Phase II trials of Campylobacter ETEC therapeutic after the US Food and Drug Administration (FDA) removed the clinical hold.





