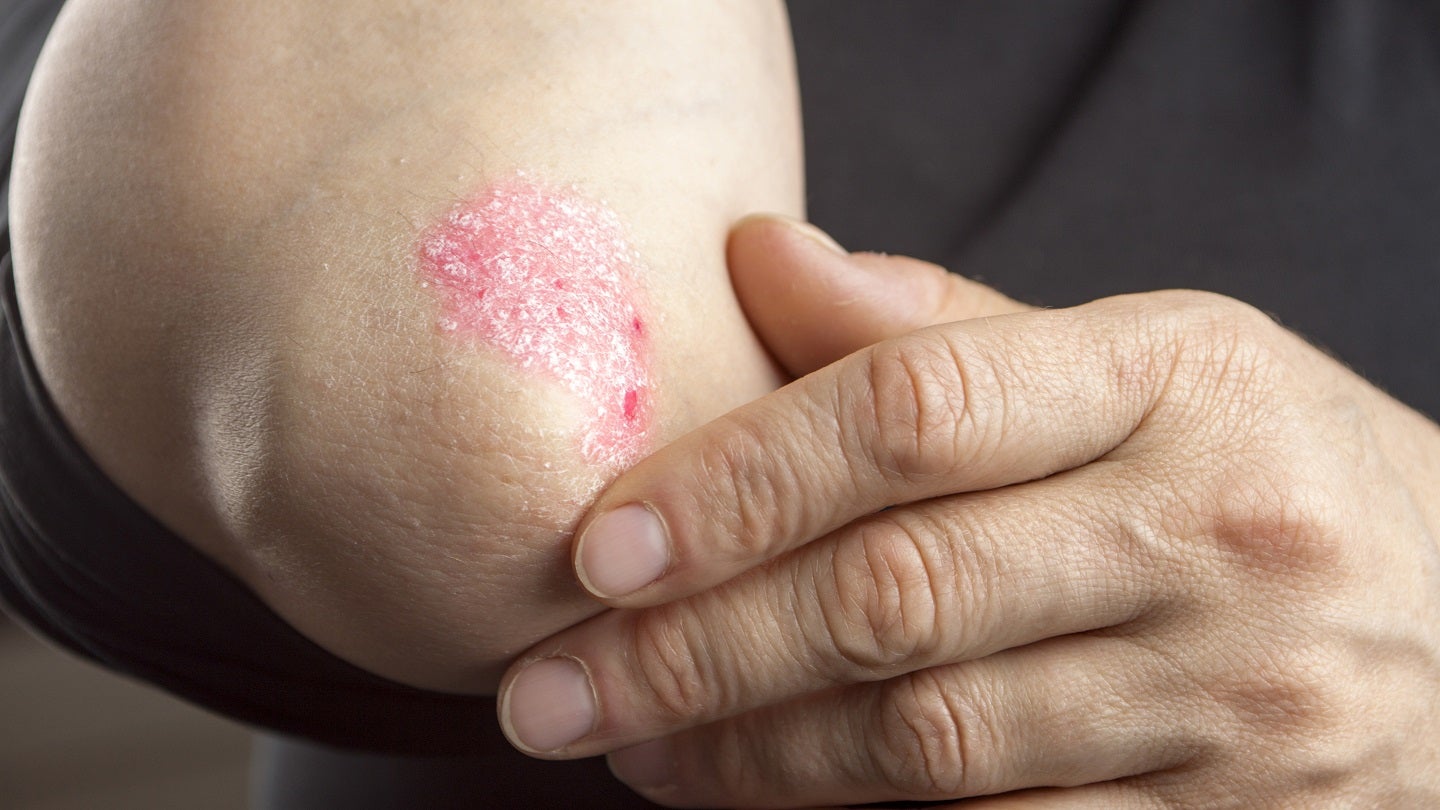
Ventyx Biosciences has completed the enrolment of patients in the Phase II trial of VTX002 in ulcerative colitis and SERENITY trial of VTX958 in plaque psoriasis.
The placebo-controlled, randomised, double-blind study of VTX002 has enrolled around 180 patients with moderately to severely active ulcerative colitis. They will be randomised to receive one of two doses of VTX002 against placebo in an induction treatment period of 13 weeks.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
They will be followed by a blinded long-term extension period of 39 weeks.
Proportion of patients achieving clinical remission at week 13, as defined by the modified Mayo Score, is the primary efficacy endpoint.
Ventyx has enrolled nearly 200 patients with moderate to severe plaque psoriasis in the dose-ranging, double-blind, placebo-controlled, randomised Phase II SERENITY trial.
Patients will be randomised to receive one of four VTX958 doses against placebo in a 16-week treatment period which will followed by a blinded long-term extension period of the same duration.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataProportion of subjects achieving a reduction of 75% in the Psoriasis Area and Severity Index (PASI 75) at week 16 is the primary efficacy endpoint.
Ventyx Biosciences president and chief medical officer Dr William Sandborn said: “We believe the robust enrolment activity in these trials demonstrates tremendous interest from patients and investigators in novel oral therapies for autoimmune diseases.”
Topline results from both the trials are expected in the fourth quarter of this year.
Ventyx is also continuing enrolment in Phase II HARMONY trial of VTX958 in patients with moderately to severely active Crohn’s disease and Phase II trial in active psoriatic arthritis. Topline results from the trials are anticipated next year.





