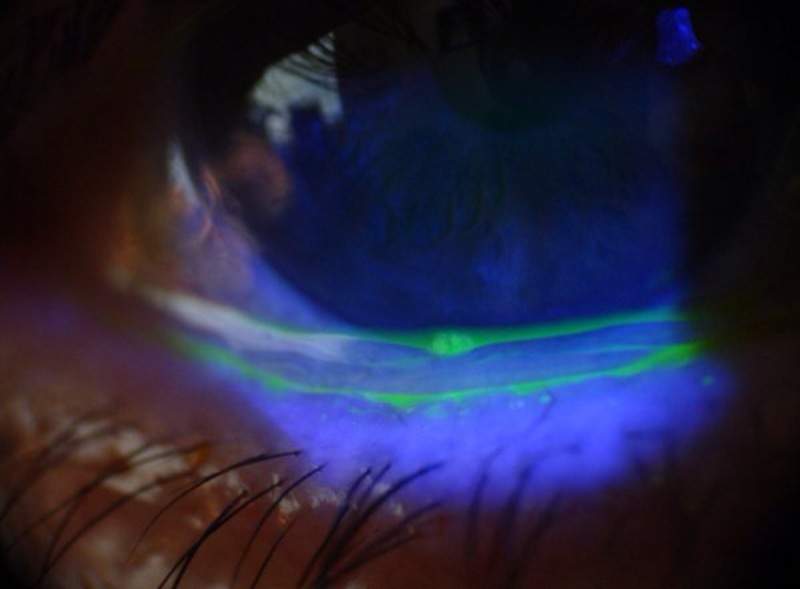
Wize Pharma has reported positive top-line results from its Phase II clinical trial evaluating LO2A for the symptomatic treatment of dry eye syndrome (DES) in patients with moderate to severe conjunctivochalasis (CCh).
The multi-centre, randomised, double-blind, placebo-controlled trial studied the efficacy and safety of LO2A in comparison with placebo.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Patients were randomly distributed in a 1:1 ratio to either the LO2A or placebo treatment group to receive topical eye drops for three months.
The results are based on the analysis of the trial’s primary endpoint, measured by the reduction in Lissamine green conjunctival staining (LGCS) score from baseline to three months.
The analysis included 49 fully evaluable patients, using a mixed model with repeated measures (MMRM) and employing all post baseline observations.
It showed statistical significance between the LO2A group and the placebo group, as well as a strong drift towards significance with average reduction in LGCS score in the LO2A and placebo groups respectively.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataFull statistical data from the trial is expected to be available once the statistical results and conclusion are available and approved.
Wize Pharma chairman Noam Danenberg said: “We believe the full results from this study will support our clinical development path and provide firm basis for presentation and discussions with the FDA for the approval pathway of LO2A in the US and additional countries.”
CCh affects up to one-third of dry eye patients in the US.





