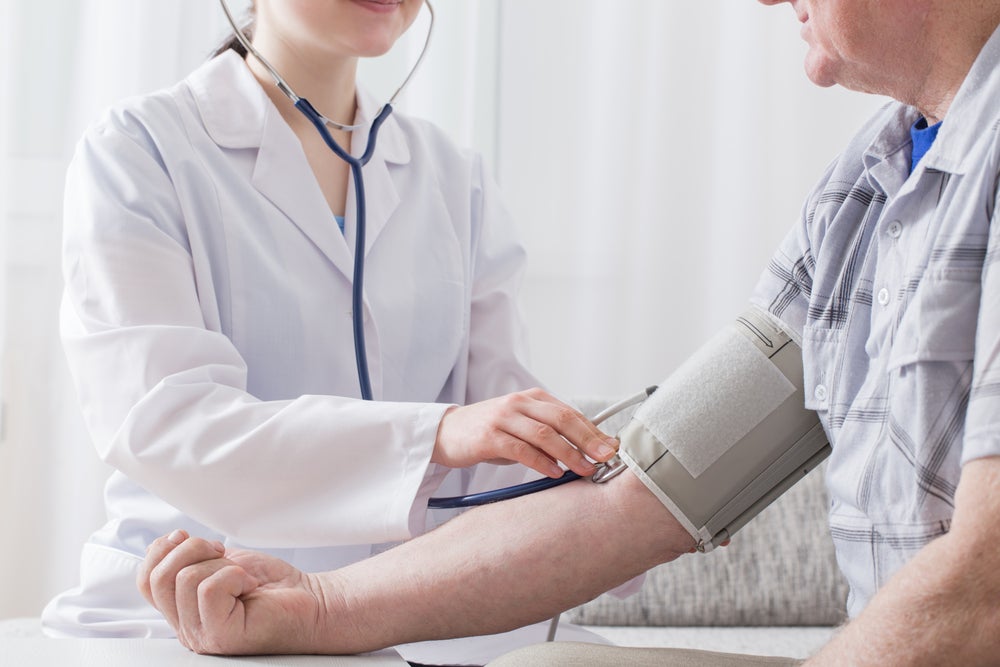
A renal denervation device from Bioheart has demonstrated positive clinical results in a Chinese study to add to research already completed in Japan.
In the prospective, multi-centre, blinded and randomised study (NCT02901704), 217 Chinese patients with primary hypertension were split into a renal denervation group and a renal angiography (sham) group.
Patients who underwent renal denervation with Bioheart’s Iberis device achieved the primary endpoint by demonstrating a reduced average 24-hour ambulatory systolic blood pressure from baseline at a three-month follow-up. These patients’ blood pressure was reduced by 11.93 mmHg compared to 2.58 mmHg in the sham group. Bioheart also reported no device-related major adverse events.
The Iberis device is a multi-electrode renal artery radiofrequency (RF) ablation catheter system which received CE marking in 2016. Renal denervation works by reducing nerve activity surrounding arteries. High energy emittance destroys these nerves which are important in controlling blood pressure.
Bioheart, which is a subsidiary of Netherlands-based Angiocare, claims it is the only renal denervation system with CE marking that can be used for transradial approach (TRA) and transfemoral approach (TFA).
Renal denervation systems for lowering blood pressure have travelled a rocky road to clinical recommendation for primary treatment. Indeed, many devices are still mid-journey, with no US Food and Drug Administration (FDA) cleared device yet. Medtronic has, however, submitted a premarket approval (PMA) package for its Symplicity Spyral device, and Metavention recently received the go-ahead for a new pivotal study. ReCor Medical has had results for its own system published in Journal of the American Medical Association (JAMA). Cordis of Johnson & Johnson has had a CE marked renal denervation system since 2014.
A market model by GlobalData forecasts the renal denervation catheter market to reach $316.2m by 2030.