The use of artificial intelligence (AI) can help improve trial efficiency in various ways said Danish Mairaj, principal engineer of medical device design at Resyca.
He cited different ways the AI can be used – for efficient trial design, increasing patient recruitment and data analysis.
The various methods that can be employed to accelerate clinical trials for medical devices including the use of AI and real-world data (RWD) were at the forefront of discussions at the Outsourcing in Clinical Trials: Medical Devices Europe 2024 conference in Munich, Germany over 30-31 January.
Mairaj highlighted that the current process for obtaining regulatory approval for a medical device was long and arduous, adding that the use of AI can not only decrease the time for gaining approval but can also improve the current 10% success rate.
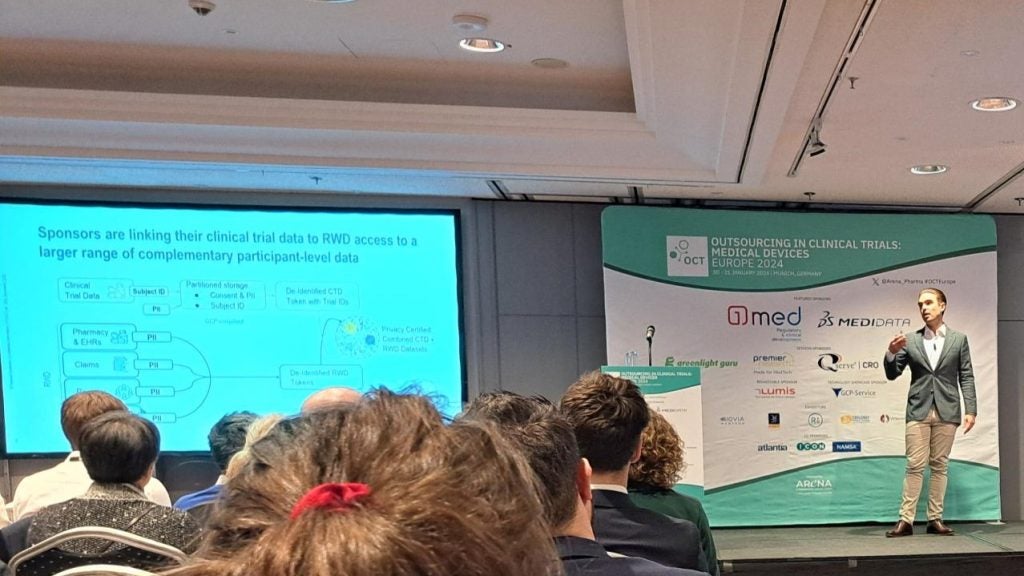
In addition, Ben McConnochie, who leads the Trial to RWD Linkage team at Medidata, talked about how combined RWD-trial datasets can significantly enhance evidence generation without adding burden to the investigators, sponsors, or patients. Adding that with the proper consent combined datasets can be used to investigate extended follow-up data post trial completion.
Ben also highlighted that combined datasets unlock novel analytics that would be impossible through purely traditional trial approaches, citing the example of how linked RWD can be used to track outcomes for patients lost to follow-ups. He concluded with stating these linkage approaches are already becoming a key part of evidence generation strategies across the industry in the US, and are being explored in other geographies.
The US Food and Drug Administration (FDA) has provided guidance on how to use RWD to supplement regulatory submission. One case study the agency provided used historical data to supplement label expansion for the therapy. In 2020, the US Food and Drug Administration (FDA) approved AstraZeneca and Merck’s Koselugo (selumetinib) for treating children with neurofibromatosis type 1 (NF1). The approval was based on a Phase II trial that used two sets of historical data from a previous natural history study of NF1 and a placebo arm of a previous clinical trial in NF1 for an entirely different drug, respectively. The data was used to form an external control arm comprised of 50 patients.