Clinical Metadata Repository

The Formedix Clinical Metadata Repository (MDR) is a centralised, all-in-one, fully integrated clinical metadata repository. You can get started straight away. It is off-the-shelf, so there is no need for complex implementation.
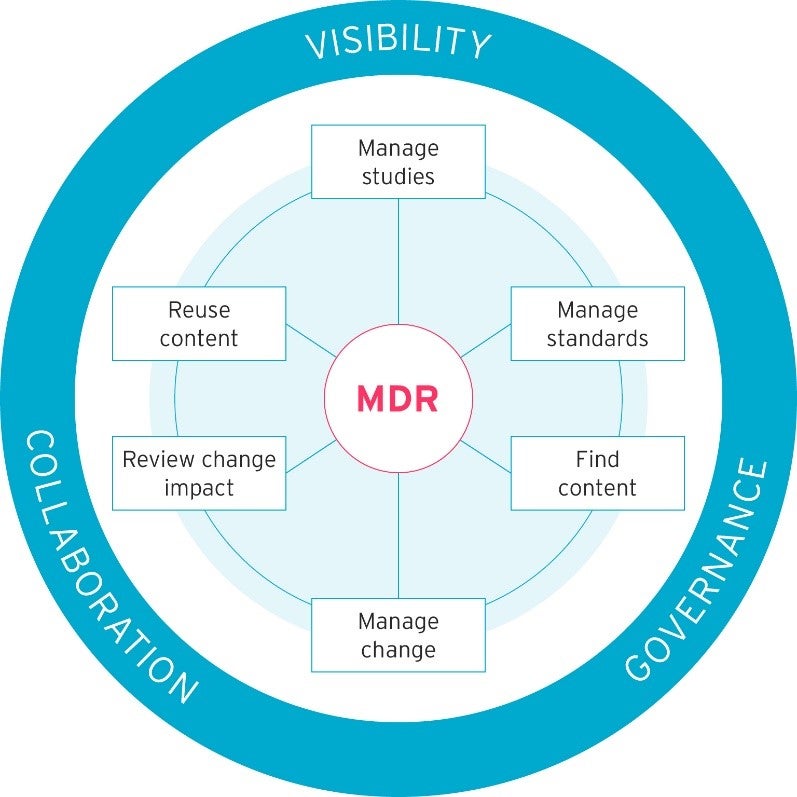
Our clinical MDR lets you manage, store, find, reuse, and share your clinical metadata content. It helps to reduce costs and manual labour and saves lots of time. You can see an overview of all its benefits in the diagram below.
Finding content
Because all your content is in one place, you do not need to go hunting for it. Formedix has a robust search function that lets you search across all your standards and studies quickly and easily. Now, there’s no need to trawl through different documents, stored in different locations.
Design and build studies faster
Formedix lets you import content from existing standards or studies, for example, from your EDC, e-clinical database, or other external systems. It is just a case of moving content from one place to another.
You can see EDC specific designs, and instantly make EDC build files. Just choose the EDC you want to work with and switch on the plugin. You can create detailed specifications for all your stakeholders and generate annotated CRFs in one click.
Reuse
Use your standardised content again and again across all your studies. So once they’re designed and approved, that is it. You can build your studies quickly, safe in the knowledge they are consistent and correct. Data quality is enhanced and there is less chance of collecting the wrong data or collecting too much.
Impact Analysis
You can measure the impact of a change before you make it. Formedix generates a report that shows you all the associated standards and standard assets that are affected. You can see what upstream and downstream metadata and processes are affected if a particular change is made. Now you are in a position to make an informed decision about whether the proposed change is worth making.
Managing change
You can easily manage change with Formedix. Team members can request changes to metadata content. You can see details of the change, such as, who requested it, why it was requested, and when. You benefit from a structured process that adheres to regulatory compliance. And in turn, reduces associated risk.
Governance
You can set up a lifecycle for your studies and standards to transition through, based on your company’s governance process. Governance and managing change is key to the evolution of your organisational standards. Making them robust leads to safer, more effective clinical trials.
Traceability
Traceability is key for allowing transparency between team members. Formedix lets you see a full and detailed history of standards. And you can see which standards are used in a specific study. You can also see who changed what, and when. So, if you need any questions answered, you know exactly who to go to.
