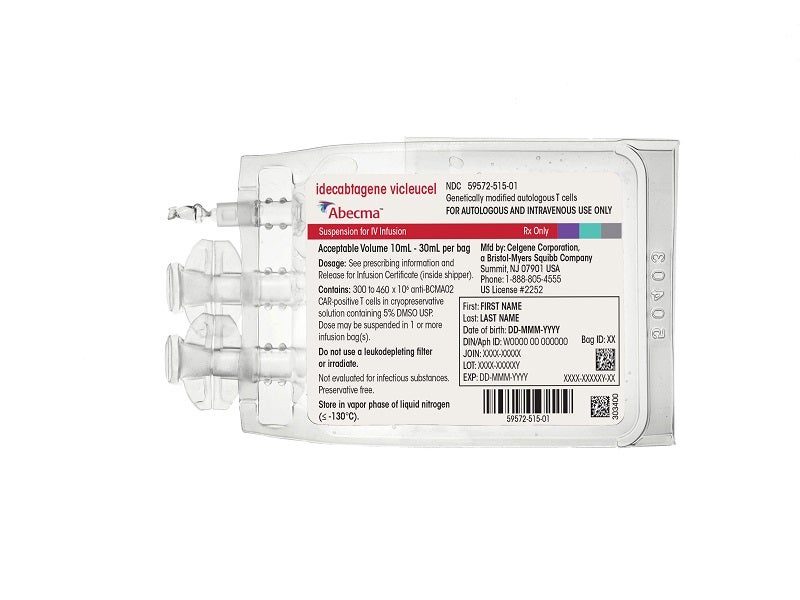
Abecma (idecabtagene vicleucel) is the first cell-based gene therapy indicated for the treatment of relapsed or refractory (r/r) multiple myeloma in adult patients, following at least three prior lines of treatment, including an immunomodulatory drug, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
Abecma is being jointly developed and marketed by Bristol-Myers Squibb (BMS) and Bluebird Bio under a co-development, co-promotion and profit share agreement in the US.
Abecma’s single dose is available as a cell suspension of 300 to 460 X 106 chimeric antigen receptor (CAR)-positive T cells in one or more infusion bags for intravenous administration. Each dose is engineered using the patient’s own T-cells, which are collected, altered genetically and infused back into the patient to recognise and kill the cancer cells vigorously.
Regulatory approvals for Abecma
In March 2020, BMS and Bluebird Bio submitted a biologics license application (BLA) for idecabtagene vicleucel (ide-cel, bb2121) to the US Food and Drug Administration (FDA) for the treatment of adult patients with multiple myeloma.
In July 2020, the companies resubmitted the BLA to the FDA for idecabtagene vicleucel with additional details on the Chemistry, Manufacturing and Controls (CMC) module, satisfying the regulatory request made by the FDA in May 2020 following the initial BLA submission. The FDA accepted the resubmitted BLA for priority review in September 2020 and approved the therapy in March 2021.
Abecma also holds orphan and breakthrough therapy designations (BTD) from the FDA and PRIority MEdicines (PRIME) designation and validation for its marketing authorisation application (MAA) by the European Medicines Agency (EMA) for r/r multiple myeloma.
In June 2021, the EMA’s Committee for Medicinal Products for Human Use (CHMP) received a positive opinion for Abecma for the treatment of adult patients with r/r multiple myeloma. The European Commission (EC) granted conditional marketing authorisation to Abecma in August 2021.
Japan’s Ministry of Health, Labour and Welfare approved the therapy for the treatment of adult patients with r/r multiple myeloma in January 2022.
Causes and symptoms of multiple myeloma
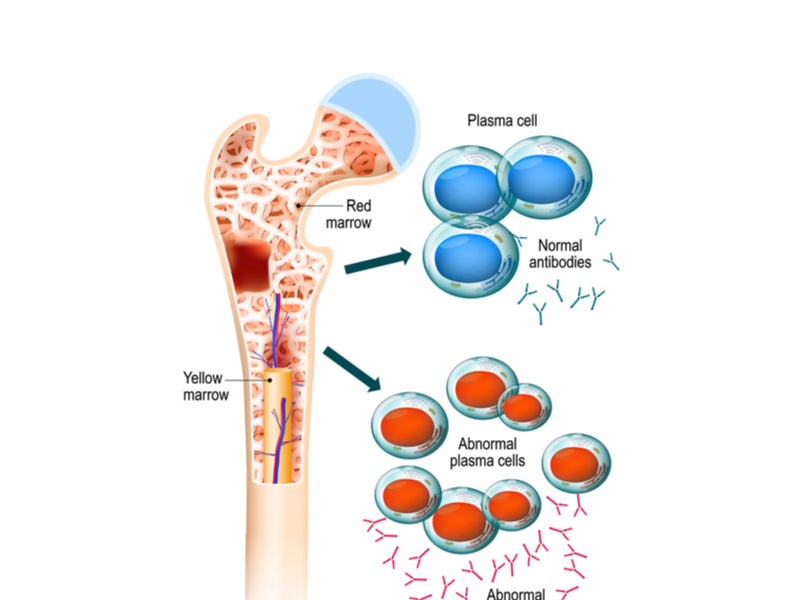
Multiple myeloma is a blood cancer that typically originates in the bone marrow and is formed by plasma cells, a type of malignant white blood cell.
Cancerous plasma cells produce aberrant proteins instead of beneficial antibodies, which can lead to complications. More than 275,000 new multiple myeloma cases are expected to occur in 2040 worldwide.
Symptoms of multiple myeloma include loss of appetite, weakness, weight loss, excessive thirst, bone pain or bone fractures, nerve damage, frequent infections, and impaired kidney function.
Abecma’s mechanism of action
Abecma is a first-in-class genetically modified autologous CAR-positive T-cell therapy directed to B-cell maturation antigen (BCMA), a protein expressed on the malignant cells in multiple myeloma.
It is a personalised immunotherapy that identifies and binds to BCMA, causing the death of the BCMA-expressing cells.
Clinical trials on Abecma
Abecma’s safety and efficacy were measured in an open-label, single-arm, multi-centre, pivotal Phase II clinical trial named KarMMa, which enrolled 127 adult patients with r/r multiple myeloma with at least three prior lines of antimyeloma therapy.
The drug’s efficacy was assessed in 100 patients, who received Abecma at doses ranging from 300 to 460 x 106 CAR-positive T cells. Among the patients assessed, 88% had four or more prior lines of treatment, while 85% were triple-class refractory.
The trial’s primary endpoints were the overall response rate (ORR), complete response, and duration of response (DOR), as determined by the Independent Response committee (IRC), using the International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma.
The ORR for the efficacy-evaluable population (n=100) was 72%, while 28% of patients achieved a complete response. The median DOR for the population treated with Abecma was 10.7 months.
Common side effects of Abecma include cytokine release syndrome (CRS), cytopaenia, fatigue, musculoskeletal pain, infections, a weakened immune system, and hypogammaglobulinaemia.