ADUHELM™ (aducanumab-avwa) is a first-of-its-kind monoclonal antibody indicated for the treatment of Alzheimer’s disease.
Developed by Swiss biopharmaceutical company Neurimmune, ADUHELM was in-licensed by US-based biotechnology company Biogen for further development in 2007. In October 2017, Biogen partnered with Japanese pharmaceutical company Eisai for the collaborative development and commercialisation of the drug worldwide.
ADUHELM is a clear to opalescent and colourless to yellow solution available in a single-dose vial of 100mg/ml solution for intravenous administration. The wholesale price of one injection administered once every four weeks is $4,312 for a patient of 74kg average weight with mild cognitive impairment (MCI) or mild dementia. The maintenance dose (10mg/kg) costs $56,000 a year.
ADUHELM approvals and controversy
In July 2020, Biogen submitted a biologics licence application (BLA) to the US Food and Drug Administration (FDA) for ADUHELM (aducanumab-avwa) injection. In June 2021, the FDA granted accelerated approval to the drug based on the reduction in amyloid-beta plaques, a characteristic biomarker of the disease, observed in patients treated with ADUHELM. It is the first new treatment for Alzheimer’s disease approved in the US in almost 20 years.
Biogen and Eisai have launched programmes in collaboration with the National Association of Free and Charitable Clinics (NAFC) and CVS Health to give all eligible patients, including the underserved communities, access to the drug. Biogen is also working on a multi-year agreement with the Veterans Health Administration (VHA) to support the access of ADUHELM for veterans throughout the VHA system.
The FDA’s approval of the drug was controversial due to its inconclusive clinical trials data. The application submitted by the company was highly complex and the clinical studies left several gaps regarding its clinical benefit.
The FDA’s advisory committee had different perspectives on ADUHELM’s clinical benefit and was not unanimous on its approval. The drug’s continued approval for the condition may be based on the verification of clinical benefit in a confirmatory trial.
Alzheimer’s disease causes and symptoms
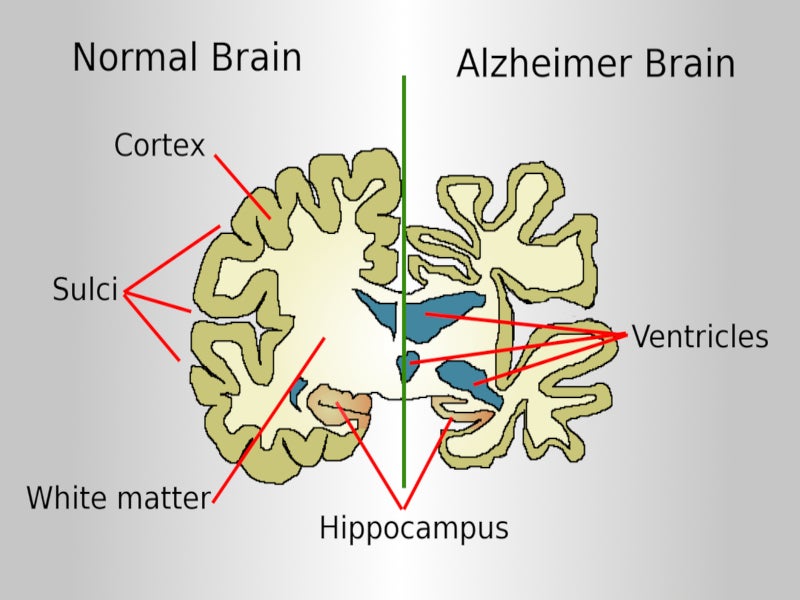
Alzheimer’s disease is a progressive neurodegenerative disorder caused by the accumulation of the amyloid-beta plaques in the brain that drive the accumulation of tau pathology and neural degeneration.
In the US, around 5.8 million people aged 65 and older suffer from Alzheimer’s disease. The cost of the lifetime care of a person with Alzheimer’s disease is estimated to be around $500,000 in the US.
Early signs of the disease include difficulty in recalling recent incidents or conversations, which worsens with time, resulting in memory loss.
ADUHELM’s mechanism of action
ADUHELM is a human, immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against the accumulated soluble and insoluble forms of amyloid-beta to reduce their levels in the brain.
Clinical studies on ADUHELM
ADUHELM’s efficacy for the treatment of Alzheimer’s disease was evaluated in two double-blind, randomised, placebo-controlled, parallel-group, phase three clinical studies, EMERGE (Study 1) and ENGAGE (Study 2), in patients with early stages of Alzheimer’s.
The studies’ primary endpoint was the delay in clinical decline in Alzheimer’s patients. EMERGE met the primary endpoint, showing a reduction in clinical decline, while ENGAGE failed to meet the primary endpoint. Despite this, ADUHELM demonstrated a consistent dose-dependent and time-dependent reduction in the levels of amyloid-beta plaques in the brain.
In EMERGE, 1,638 patients were randomised at a 1:1:1 ratio to receive either a low dose of ADUHELM, a high dose of ADUHELM, or a placebo. Out of 1,638 patients, 488 were evaluated in the amyloid PET sub-study, of which 302 were evaluated at week 78.
At week 78, the ADUHELM high dose group showed a reduction in clinical decline compared to the placebo group. Around 71% of ADUHELM-treated patients demonstrated a consistent dose-dependent and time-dependent effect on the reduction of amyloid-beta plaques.
In ENGAGE, 1,647 patients were randomised at a 1:1:1 ratio to receive either a low dose of ADUHELM, a high dose of ADUHELM, or a placebo. The amyloid PET sub-study included 585 patients, 374 of whom were evaluated at week 78.
At week 78, no statistically significant differences were observed between ADUHELM-treated patients and placebo-treated patients. Around 59% of ADUHELM-treated patients demonstrated a consistent dose-dependent and time-dependent effect on the reduction of amyloid-beta plaques.
In another exploratory, double-blind, randomised, placebo-controlled, dose-ranging Phase 1b study, PRIME, 197 patients were randomised to receive 10mg/kg of ADUHELM or a placebo. Around 61% of ADUHELM-treated patients showed a consistent dose-dependent and time-dependent effect on the reduction of amyloid-beta plaques.
ADUHELM’s safety profile was established in more than 3,000 patients who received at least a single dose of the drug.
Amyloid-related imaging abnormalities (ARIA) were the most frequently reported adverse event during the radiographic detection of events.