Agamree (vamorolone) is a first-in-class steroidal drug to treat Duchenne muscular dystrophy (DMD) in patients aged two years and older.
US-based pharmaceutical company ReveraGen BioPharma developed the drug and granted an exclusive license to Santhera Pharmaceuticals, a Swiss pharmaceutical company, for the development and commercialisation of vamorolone for all indications globally in September 2020.
Santhera out-licenced the rights to vamorolone in China to Sperogenix Therapeutics in January 2022 while the North America rights were out-licensed to Catalyst Pharmaceuticals in July 2023. Sperogenix is a pharmaceutical company based in China while Catalyst Pharmaceuticals is a biopharmaceutical business based in the US.
Agamree is available in 40mg/ml dosage strength as an orange-flavoured white to off-white suspension for oral administration.
Catalyst has scheduled the commercial launch of the drug in the US in the first quarter of 2024, supported by its Catalyst Pathway Program.
Regulatory approvals for Agamree
The US’ FDA approved Agamree for treating DMD in October 2023. In the same month, the European Medicines Agency’s Committee for Medicinal Products for Human Use provided a positive opinion for the drug to treat DMD in children and adults aged four years and older.
Agamree will be the first drug to receive complete approval in Europe for the treatment of individuals with DMD, if approved by the European Commission.
Agamree holds orphan drug and rare paediatric disease designations statuses in the US and Promising Innovative Medicine status from the UK Medicines and Healthcare Products Regulatory Agency for DMD. The drug also has orphan drug status in Europe.
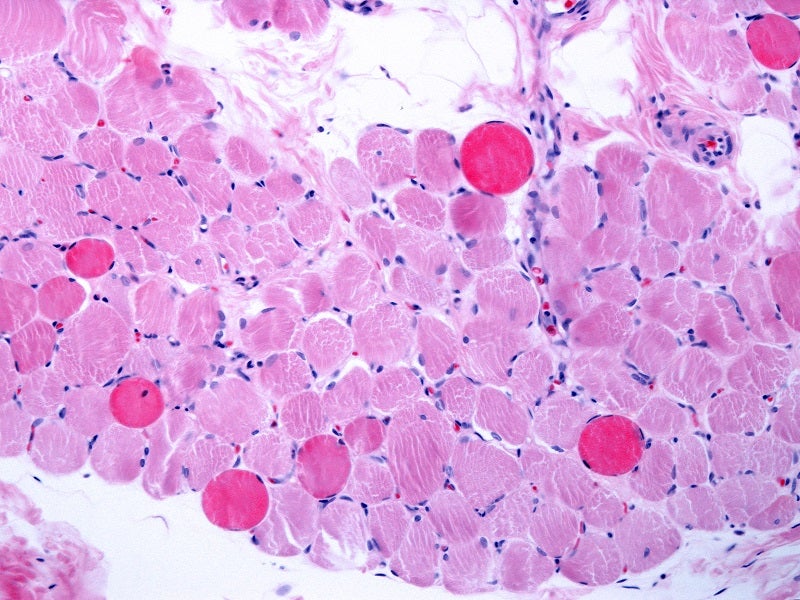
DMD causes and symptoms
DMD is a rare and severe neuromuscular disorder caused by mutations in the dystrophin gene. It causes neuromuscular inflammation characterised by progressive skeletal, pulmonary, and cardiac muscular impairment.
The condition may lead to a progressive loss of mobility by adolescence, requiring a wheelchair.
Cardiac and respiratory issues begin in the teenage years and progress to severe, life-threatening complications. Early signs of DMD include frequent falls, difficulty rising from a lying or sitting position, running and jumping problems, waddling gait, toe walking, muscle pain and stiffness, learning disabilities, and delayed growth.
DMD affects an estimated 11,000 to 13,000 individuals in the US.
Agamree’s mechanism of action
Vamorolone is a dissociative steroid that selectively binds to the glucocorticoid receptor to exert anti-inflammatory and immunosuppressive effects. Vamorolone also inhibits mineralocorticoid receptor activation by aldosterone.
It is a first-in-class medication that binds to the same receptors as corticosteroids and modifies their downstream activity.
The precise mechanism by that vamorolone exerts its therapeutic effects in patients with DMD is unknown, though.
Clinical trials on Agamree
The FDA’s approval of Agamree was based on data from the pivotal Phase IIb VISION-DMD clinical trial, supplemented by safety information gathered from three open-label studies, including extension studies.
VISION-DMD is a multicentred, randomised, double-blinded, parallel-group, placebo and active-controlled, multinational study including a double-blind 24-week period to demonstrate the efficacy and safety of vamorolone versus placebo and prednisone, followed by a 24-week period to determine the maintenance of efficacy and obtain additional safety and tolerability data in the longer term.
The study enrolled 121 male DMD patients aged from four years to less than seven years.
The primary endpoint was the change from baseline in Time to Stand from supine position (TTSTAND) velocity in patients treated with Agamree 6mg/kg a day compared to placebo at 24 weeks. TTSTAND velocity assesses muscle function by measuring the duration it takes for a patient to transition from a supine position on the floor to an upright standing position.
The study achieved the primary endpoint by demonstrating the superiority of vamorolone at a dosage of 6mg/kg/day over placebo in the TTSTAND velocity test at 24 weeks of treatment and demonstrated a good safety and tolerability profile.
The most common adverse events reported during the study were cushingoid features, vomiting, vitamin D deficiency, agitation, anxiety, irritability, mood alteration, sleep disorder, and stereotypy.