Amondys 45 (casimersen) injection is an antisense oligonucleotide indicated for the treatment of patients with Duchenne muscular dystrophy (DMD). It is the first US Food and Drug Administration (FDA) approved treatment for DMD patients with a mutation amenable to skipping exon 45.
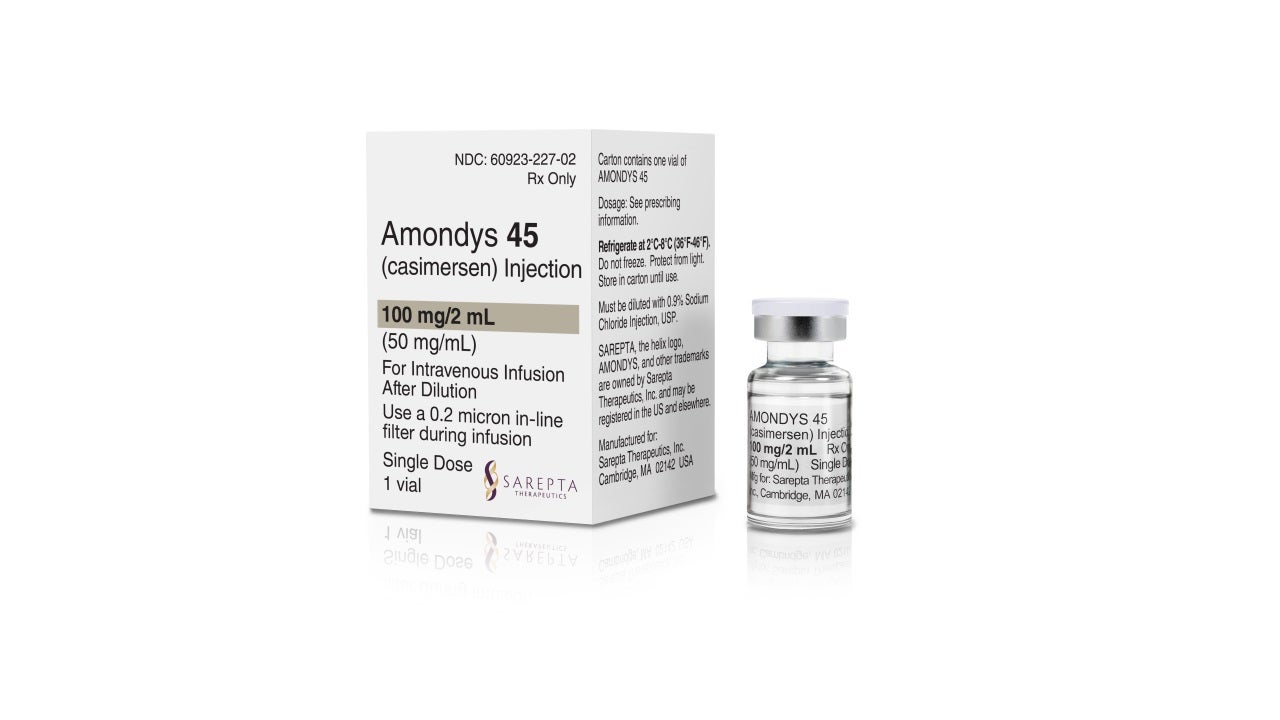
Developed by Sarepta Therapeutics, Amondys 45 is available as a 100mg/2ml (50mg/ml) clear to slightly opalescent solution in a single-dose vial for intravenous administration.
Amondys 45 is a colourless liquid and may contain small quantities of small, white to off-white amorphous particles.
Amondys 45 approvals
In June 2020, Sarepta Therapeutics submitted a New Drug Application (NDA) for casimersen to the FDA. The NDA was accepted by the FDA in August 2020.
In February 2021, the FDA granted accelerated approval to Amondys 45 for the treatment of DMD in patients with a confirmed dystrophin gene mutation that is amenable to exon 45 skipping.
In June 2019, the drug received orphan drug designation from the FDA for the treatment of DMD.
The approval of the casimersen injection marks Sarepta’s third approved RNA exon-skipping therapy for DMD patients. The company’s other two DMD drugs are Exondys 51 and Vyondys 53, which are indicated for the treatment of DMD patients amenable to exon 51 and exon 53 skipping respectively.
Amondys 45 is anticipated to target around 8% of DMD patients with mutations amenable to exon 45 skipping.
DMD causes and symptoms
DMD is a rare genetic neuromuscular disorder that mainly affects the male population. It is the most common form of muscular dystrophy in children and occurs due to genetic mutations that prevent the body from producing dystrophin, which is key for the proper functioning of muscles.
The DMD gene encodes instructions for producing dystrophin, a protein found in muscle fibre. The protein plays an essential role in maintaining muscle health and its absence causes the muscle cells to weaken and degenerate over time.
Characterised by progressive muscle deterioration, DMD affects around one in every 3,600 male infants in the world.
The common signs and symptoms of DMD include fatigue, inability to walk until around 18 months of age, delayed speech, walking on toes with legs apart, walking with the belly pointed out, behaviour and learning problems, and larger calves when compared to other children of the same age or size.
Children diagnosed with DMD may also have conditions such as epilepsy and other neuropsychiatric disorders.
Casimersen mechanism of action
Casimersen is an antisense phosphorodiamidate morpholino oligonucleotide designed to bind to exon 45 of the dystrophin pre-mRNA, leading to the exon being skipped during mRNA processing in patients whose genetic mutations are amenable to exon 45 skipping.
The drug utilises Sarepta’s proprietary phosphorodiamidate morpholino oligomer (PMO) chemistry and exon-skipping technology to skip exon 45 of the DMD gene. Skipping exon 45 allows an internally truncated dystrophin protein to be produced.
Clinical trials on Amondys 45
The FDA’s approval of Amondys 45 is based on the outcome of an ongoing ESSENCE clinical trial, a global, double-blind, multi-centre study to evaluate the efficacy and safety of casimersen and golodirsen.
The placebo-controlled study evaluated the efficacy and safety of casimersen in male patients, aged between seven and 20 years, with DMD and a confirmed genetic mutation amenable to exon 45 skipping.
In the trial, 43 DMD patients were randomised at a 2:1 ratio to receive either a once-weekly intravenous infusion of casimersen dosed at 30mg/kg or a placebo, for up to 96 weeks.
Interim results from the study demonstrated that the patients who received Amondys 45 had a greater increase in levels of dystrophin from baseline to week 48 of treatment compared to patients who received the placebo.
The most common adverse reactions observed during the clinical study were upper respiratory tract infections, headache, fever, joint pain, cough, and pain in the mouth and throat.