Aphexda™ (motixafortide) is a chemokine receptor CXCR4 antagonist indicated in combination with filgrastim (G-CSF) to mobilise hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma.
Developed by BioLineRx, a biopharmaceutical company based in Israel, Aphexda is available as a white-to-off-white lyophilised powder in a single-dose vial for reconstitution in 62mg dosage strength for subcutaneous injection.
Regulatory approvals for Aphexda
BioLineRx submitted a new drug application (NDA) for Aphexda in stem cell mobilisation for autologous bone marrow transplantation for multiple myeloma patients to the US Food and Drug Administration (FDA) in September 2022.
In November 2022, the FDA accepted the NDA for review and filed it for Aphexda in stem cell mobilisation for autologous transplantation in multiple myeloma patients. The drug was approved for use in combination with G-CSF for the condition in September 2023.
The drug holds orphan drug designation for the treatment of pancreatic cancer in the US and Europe.
Multiple myeloma causes and symptoms
Multiple myeloma is an incurable blood cancer that affects plasma cells, a type of white blood cell found in the bone marrow.
It is estimated that more than 35,000 people will be diagnosed with the disease and 13,000 will die due to the condition in the US in 2023, according to the American Cancer Society.
The symptoms of the disease include bone pain or fracture, low red blood cell counts, fatigue, high calcium levels and kidney problems or infections.
Treatment options include autologous stem cell transplantation (ASCT), which is considered a part of the standard treatment model for several blood cancers including multiple myeloma. The current ASCT practice contains four to six induction therapy cycles following the stem cell mobilisation process.
The successful outcomes of ASCT are based on the sufficient mobilisation of the stem cells during the treatment process.
Aphexda’s mechanism of action
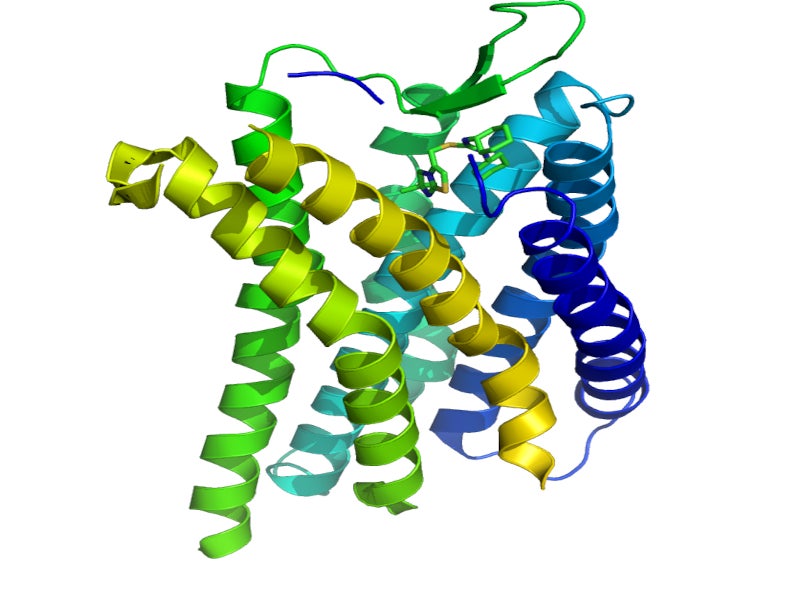
Motixafortide is an inhibitor of the CXCR4 receptor, which blocks the binding of its cognate ligand, C-X-C motif chemokine ligand 12 (CXCL12).
CXCL12 and CXCR4 play a part in the movement of human hematopoietic stem cells away and back into the marrow compartment. Motixafortide demonstrated leukocytosis and an increase in circulating hematopoietic stem and progenitor cells into the peripheral circulation in mice, rats, dogs and even humans.
The drug’s long receptor occupancy allows the mobilisation of hematopoietic stem cells to the peripheral blood for collection and transplantation in patients with multiple myeloma.
Clinical trials of Aphexda
The FDA approval of Aphexda is based on the outcomes of the second part of the Phase III GENESIS clinical trial.
GENESIS is a randomised, double-blinded, placebo-controlled study to evaluate the safety and efficacy of Aphexda in combination with G-CSF, compared to placebo plus G-CSF, for the mobilisation of hematopoietic stem cells for autologous transplantation in patients with multiple myeloma.
The first part of the study was a single-centred, lead-in, open-label study involving 12 patients receiving motixafortide plus G-CSF designed for dose determination, while the second part involved 122 patients who were randomised in a 2:1 ratio in a double-blinded, placebo-controlled and multi-centre study.
The primary objective of the study was to determine the superiority of a single dose of motixafortide plus G-CSF compared to placebo plus G-CSF for mobilising equal to or more than six million CD34+ cells in up to two apheresis sessions.
The study demonstrated that 67.5% of patients with Aphexda plus G-CSF achieved the stem cell collection goal of equal to or more than six million CD34+ cells/kg within two apheresis sessions compared to 9.5% for the placebo plus G-CSF regimen evaluated by a central laboratory.
92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the Aphexda arm and 21.4% in the placebo arm, as determined by local laboratories.
The safety of the drug in the study was determined in 92 patients who were administered Aphexda 1.25mg/kg subcutaneously plus G-CSF, and 42 patients who received placebo plus G-CSF.
Serious adverse reactions were reported in 5.4% of patients receiving Aphexda plus G-CSF.
Injection site reactions, pruritus, flushing and back pain were the most common adverse reactions observed in patients during the study.
Clinical trials of motixafortide for additional conditions
The company is conducting clinical trials on motixafortide as monotherapy and in combination with natalizumab for stem cell mobilisation for gene therapies in sickle cell disease in collaboration with Washington University School of Medicine in St Louis, Missouri, US.
In collaboration with Columbia University, the company is also advancing a major development programme for motixafortide in first-line metastatic cancer patients.