Apretude (cabotegravir) is the first and only injectable long-acting pre-exposure prevention (PrEP) treatment to minimise the risk of sexually acquired HIV-1.
The drug was developed by ViiV Healthcare, a joint venture between GlaxoSmithKline (GSK), Pfizer and Shionogi. It is approved for the treatment of HIV PrEP in adults and adolescents weighing at least 35kg and who have tested negative for HIV-1 before taking the drug.
Apretude is available as a white to light pink injectable suspension in a single-dose vial of 600mg/3ml (200mg/ml) cabotegravir. It is administered by a healthcare professional every two months following two initiation injections one month apart and an optional oral lead-in to determine tolerability.
Regulatory approvals for Apretude
In November 2020, the US Food and Drug Administration (FDA) granted breakthrough therapy designation for long-acting cabotegravir for HIV PrEP. A new drug application (NDA) for investigational cabotegravir was submitted to the FDA in May 2021 and accepted for priority review in September 2021.
Apretude received FDA approval for a PrEP option to reduce the risk of sexually acquired HIV-1 in December 2021. ViiV Healthcare has also initiated regulatory submissions to various regulatory bodies worldwide.
The drug is expected to be made available in the US in early 2022.
HIV causes and symptoms
HIV is a life-threatening condition that compromises the immune system and can lead to acquired immunodeficiency syndrome (AIDS) if left untreated. An estimated 38 million people are living with the virus worldwide, with 1.7 million new cases diagnosed each year.
Signs and symptoms of the disease include headache, rash, swollen lymph glands, weight loss, night sweats, diarrhoea, muscle aches, joint pain, fever, cough, sore throat, and painful mouth sores.
PrEP is an effective way of reducing new HIV cases in combination with successful HIV antiretroviral treatment. It decreases the risk of contracting HIV through intercourse by about 99% and from injectable drug usage by at least 74%.
Despite the widespread availability of daily oral PrEP, its use can be limited by irregular adherence, as well as cultural and structural hurdles that contribute to underutilisation in vulnerable groups.
Apretude’s mechanism of action
Apretude is a long-acting HIV-1 antiretroviral drug. It is an integrase strand transfer inhibitor (INSTI). It inhibits the replication of HIV integrase by binding to the active site and blocking viral DNA from integrating into the genetic material of human immune cells or T-cells (strand transfer stage).
The strand transfer stage is critical in the HIV replication cycle and is responsible for chronic disease development.
Clinical trials on Apretude
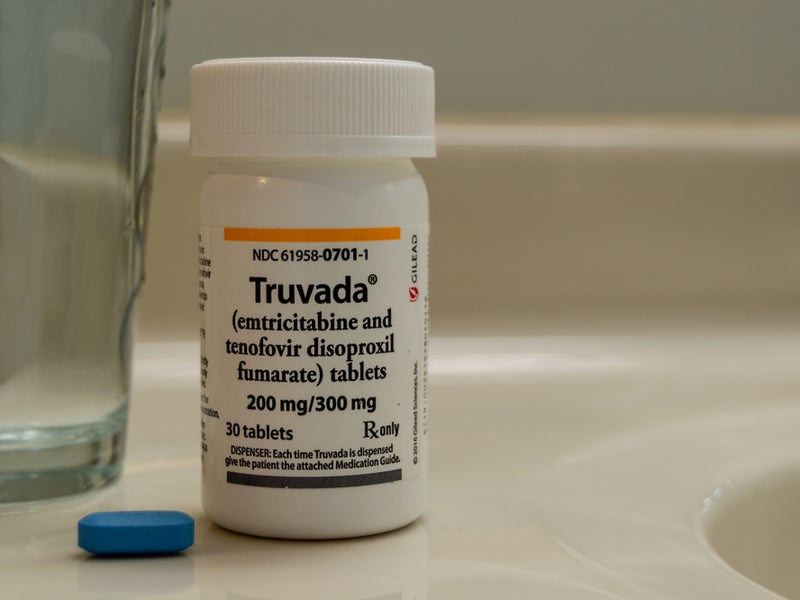
The efficacy and safety of Apretude in the treatment of HIV-1 PrEP was evaluated in two randomised, double-blind active-controlled Phase IIb/III multicentre trials named HPTN 083 and HPTN 084, which compared Apretude with Truvada.
HPTN 083 is a non-inferiority study that enrolled 4,566 uninfected cisgender men and transgender women who showed high-risk behaviour of acquiring HIV-1 infection. HPTN 084 is a superiority study that enrolled 3,224 uninfected cisgender women at increased risk of acquiring HIV-1. The trials were designed to include an oral lead-in phase to evaluate tolerability to cabotegravir before administering the intramuscular injection.
Participants who were randomised to receive Apretude began the trial with 30mg oral tablet of cabotegravir and placebo every day for up to five weeks, followed by Apretude 600mg injections in the first two months and every two months thereafter along with a placebo tablet every day.
Participants who were randomised to receive Truvada initiated the trial by taking oral Truvada and placebo every day for up to five weeks, followed by intramuscular Truvada and placebo injections in the first and second months and every two months thereafter.
The primary outcome measure of both trials was the rate of HIV infections among the participants.
The HPTN 083 study demonstrated that participants who were administered Apretude had a 69% lower risk of HIV infection compared with those given Truvada, while the HPTN 083 study demonstrated that participants given Apretude had a 90% lower risk of HIV infection compared with those who received Truvada.
The most frequent adverse reactions observed in participants taking Apretude were injection site reactions, diarrhoea, pyrexia, fatigue, sleep disturbances, nausea, stomach pain, vomiting, myalgia, reduced appetite, back pain, and upper respiratory tract infection. Adverse reactions led to the withdrawal of 6% of HPTN 083 participants and 1% of HPTN 084 participants.