BESPONSA® is an intravenous infusion indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukaemia (ALL).
The drug was discovered by Wyeth Pharmaceuticals, which is a subsidiary of Pfizer and Celltech.
Pfizer’s biologics license application (BLA) for BESPONSA® was accepted for review by the US Food and Drug Administration (FDA) in February 2017, and was approved in August of the same year.
The drug received marketing authorisation approval (MAA) from the European Commission (EC) in June 2017.
Acute lymphoblastic leukaemia causes and symptoms
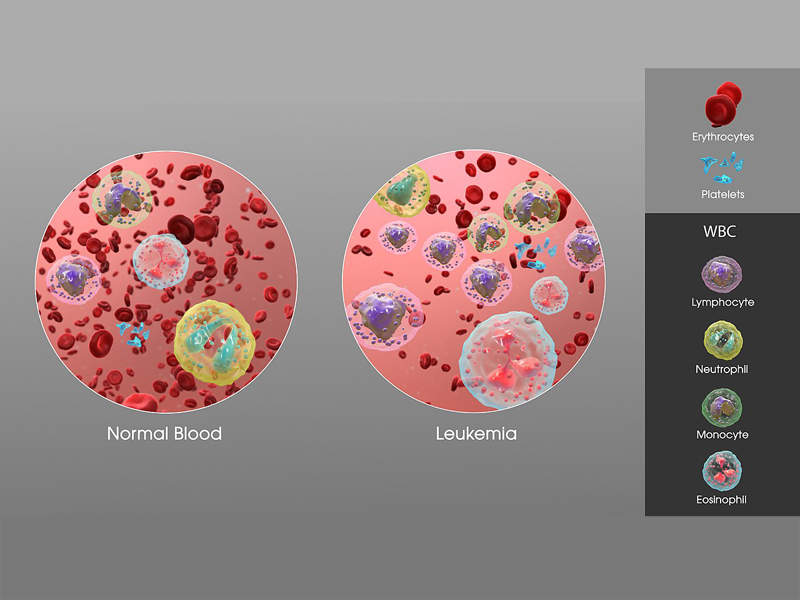
ALL is a type of cancer that causes uncontrolled growth of white blood cells in bone marrow, which prevents the production of red blood cells and platelets.
The most common symptoms of the disease include pale skin, bone and joint pain, unusual and frequent bleeding, and swollen lymph nodes.
Acute lymphoblastic leukaemia is more prevalent in children and mostly occurs in males than females. More than 650 people are diagnosed with the condition each year in the UK.
BESPONSA mechanism of action
Inotuzumab ozogamicin contained in BESPONSA® is an antibody-drug conjugate (ADC) containing a monoclonal antibody (mAb) that targets CD22, a cell-surface antigen found in cancer cells. It also consists of N-acetyl-gamma-calicheamicin, a cytotoxic agent that is covalently attached to the antibody.
BESPONSA® binds to the CD22 cell-surface antigen on B-cells causing internalisation of the ADC-CD22 complex, where the calicheamicin cytotoxic agent is released to destroy the cancer cell.
The drug is available as a 0.9mg lyophilised powder in a single-dose vial.
Clinical trials on BESPONSA
The US FDA’s approval for BESPONSA® was based on data gathered from the Phase III INO-VATE ALL trial. This open-label, randomised trial compared the safety and efficacy of BESPONSA® with chemotherapy.
A total of 326 patients with relapsed or refractory B-cell ALL and a median age of 46 years were enrolled for the study. The primary endpoints were complete remission rate (CRR) and overall survival (OS).
Results from the study showed that the CRR for patients randomised to BESPONSA® was 81%, compared to 29% for patients treated with chemotherapy. In addition, 48% of patients randomised with BESPONSA® proceeded to hematopoietic stem cell transplantation (HSCT), compared to 22% of patients treated with chemotherapy.
The median OS time for patients treated with BESPONSA® was approximately seven months, while it was more than six months for patients treated with chemotherapy.
The results also showed high rates of haematological remission and absence of minimal residual disease in patients treated with inotuzumab ozogamicin, compared to those treated with chemotherapy.
The most common adverse reactions associated with the use of BESPONSA® were thrombocytopenia, neutropenia, leukopenia, fatigue, pyrexia, nausea and headaches. Increased transaminases, abdominal pain and hyperbilirubinemia were also reported in some patients.
Marketing commentary on Pfizer
Based in the US, Pfizer is a biopharmaceutical company that specialises in the discovery and development of new and innovative solutions to prevent and treat diseases and address healthcare challenges.
Pfizer Oncology provides innovative treatments in oncology and accessible breakthrough medicines to patients suffering from cancer.