Samaritan Pharmaceuticals’ caprospinol (SP-233) is an investigational
agent under development for the treatment of Alzheimer’s disease (AD).
Towards the end of 2006, Samaritan submitted an investigational new drug (IND)
application to the US FDA to seek approval to begin clinical testing of its new
drug.
In December 2006, the FDA requested Samaritan to present more information pertaining to the drug before taking up the first phase of clinical study. The company has partnered with India-based Advinus Therapeutics to provide the requisite information to the FDA.
Caprospinol is the company’s lead compound in a series of
investigational agents being developed for AD and other neurodegenerative
disorders. It is designed to target the toxic beta-amyloid plaques that develop
in the brains of AD patients. After drugs that target cholinergic
neurotransmission, anti-amyloid therapies represent the next largest group of
compounds in development for AD.
Although the use of SP-233 has been focused principally on the treatment of Alzheimer’s disease, Samaritan claims that the drug can potentially treat Parkinson’s disease and neurological disorder.
Caprospinol may help to restore memory
Recent studies conducted by Samaritan on Caprospinol have demonstrated the drug’s ability in restoring memory. Given that it also protects against future memory impairment, the memory restoration may add much value to Caprospinol. So far, studies conducted on rats have exhibited instances of memory restoration.
Towards drugs with disease-modifying effects
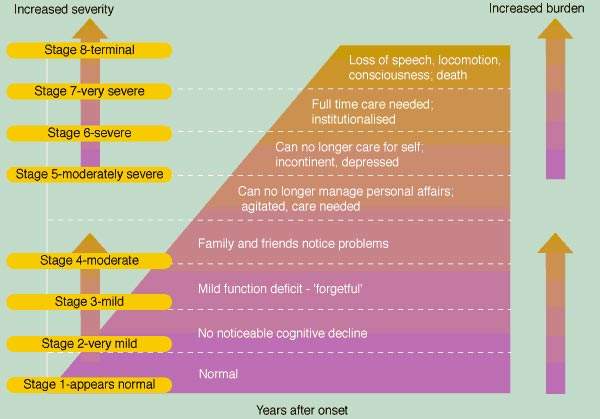
AD is a degenerative disease of the brain characterised by the progressive
loss of cortical neurones and synapses leading to a loss of memory and
cognition and accompanying functional impairment.
At post-mortem, the neocortex
and hippocampus regions of the brains of AD patients show evidence of amyloid
plaques, sometimes termed neuritic or senile plaques, as well as
neurofibrillary tangles due to hyperphosphorylation of Tau protein.
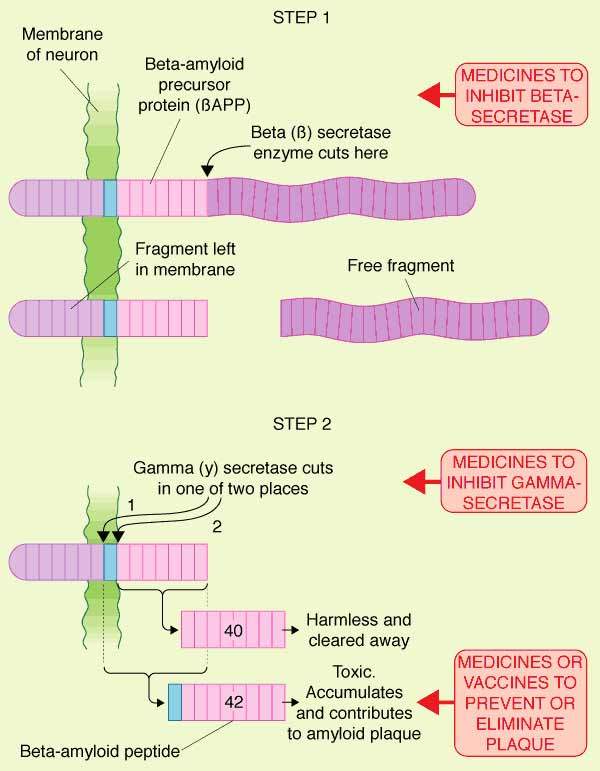
The discovery that senile plaques in the brains of AD patients contained an
accumulation of insoluble toxic beta-amyloid (β-amyloid42, derived from amyloid
precursor protein (APP), led to the search for compounds that could either prevent the deposition of toxic
beta-amyloid in the brain or eliminate existing plaques.
It is hoped that by
targeting what is believed to be a key step in the pathogenesis of AD, such
compounds will have disease-modifying effects.
Caprospinol may protect against ß-amyloid-induced toxicity
Caprospinol (SP-233) is a spirostenol (steroid derivative) that has shown
evidence of protecting neuronal mitochondria from beta-amyloid-induced cytotoxicity and cell death in
preclinical studies. Mitochondria, cytoplasmic organelles that are critical to
aerobic respiration, and needed to maintain neuronal cell energy levels.
Impaired mitochondrial functioning is seen in several CNS diseases including
AD.
Caprospinol (SP-233) is believed to act by binding to beta-amyloid peptide
and preventing its oligomerisation and entry into neurones.
Oligomerisation of beta-amyloid is an early step in the formation of senile
plaques considered the primary pathological feature of AD.
In addition to caprospinol (SP-233), Samaritan Pharmaceutical’s
pipeline also contains two other drugs in development for AD, SP-004 and
SP-008, and two stem cell neuron differentiation therapies SP-sc4 and SP-sc7.
The latter are designed to induce dormant brain cells to differentiate rapidly
into adult neurones. All of these drugs are in preclinical stage.
Combating the rising prevalence of AD
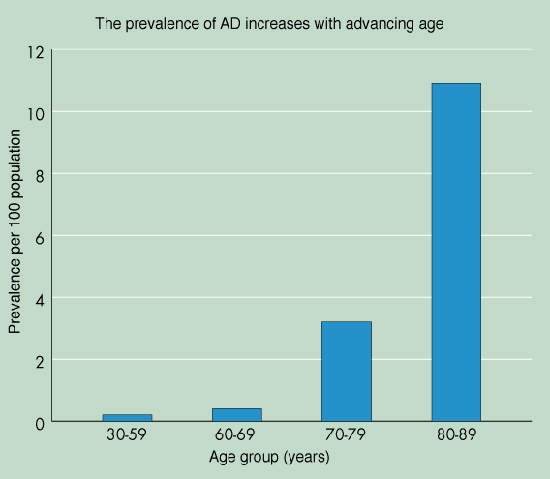
Estimates suggest that some 12–15 million people in the world have AD,
4.5 million in the US alone. Prevalence of AD is strongly age-related; in those
aged 60 years and older prevalence doubles every five years. With the growing
proportion of elderly and very elderly (>85 years) in the world’s
population, prevalence will continue rising. By 2025 it is estimated that there
will be more than twice as many people with AD than there are today, unless
more effective treatments can be developed.
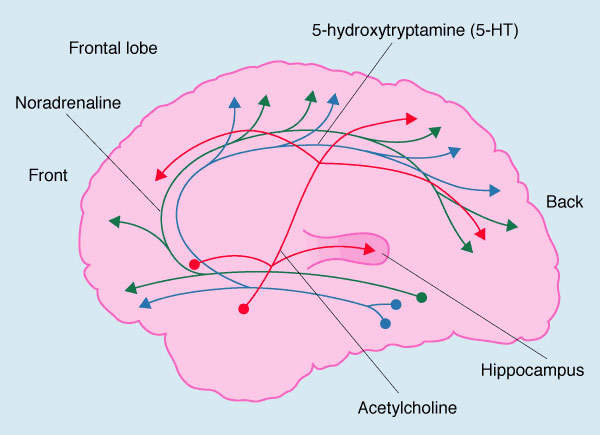
Today the treatment of AD is dominated by the use of acetylcholinesterase
inhibitors, which are designed to raise the concentration of acetycholine at
sites of neurotransmission in the cerebral cortex and forebrain. These drugs,
three of which are clinically approved, provide modest symptomatic improvement
to AD patients in the mild to moderate stage of the disease.
However, they do not address the underlying pathology of AD. Moreover, their
effects on cognition, executive functioning and behaviour are only temporary.
Of currently approved anti-dementia drugs, none have disease-modifying effects
that can halt the progression of the disease and stop cognitive decline.
New drugs with genuine disease-modifying effects are urgently required to
stop the rising prevalence of AD, the most common form of dementia to afflict
the elderly. Drugs targeted at central cholinergic dysfunction and toxic
beta-amyloid peptide represent two major therapeutic approaches for AD, others
include:
- Serotonergic agents
- Dopaminergic agents
- Glutamatergic agents
- GABA agents
- Neurotropic/neuroprotective agents
- Anti-inflammatory drugs
- Antioxidants
- Endogenous neurotrophins.
Marketing commentary
Still in its infancy, the current market for anti-dementia therapies has
amongst the highest growth dynamics in the CNS market, driven by a growing
elderly population and unmet clinical need.
Samaritan Pharmaceuticals’ caprospinol (SP-233) is about to enter a
phase I maximum dose and safety study and is thus in the earliest stage of
clinical development. It remains to be seen whether it will progress into phase
II and then pivotal phase III trials but the preclinical data look
encouraging.