Contrave (Bupropion / naltrexone) is a drug indicated for treating obesity. The drug was developed by Orexigen Therapeutics.
Orexigen submitted a New Drug Application (NDA) to the US Food and Drug Administration (FDA) on 31 March 2010.
The FDA issued a complete response letter in February 2011, asking for additional data to prove that the adverse cardiovascular events in obese patients treated with naltrexone/bupropion do not affect the benefit-risk profile of the drug. The regulator’s Division of Metabolic and Endocrinologic Products stated that it would not consider approving the drug until Orexigen provides the results of additional cardiovascular trials.
Orexigen met FDA officials in September 2011 to identify a clear path for approval of Contrave. The outcome of the meeting included a written response on the need to conduct a cardiovascular outcome trial.
In February 2012, Orexigen reached an agreement with the FDA to conduct a Contrave cardiovascular outcomes trial under a Special Protocol Assessment (SPA).
Orexigen submitted Marketing Authorisation Application (MAA) for Contrave to the European Medicines Agency (EMA) for the management of obesity in October 2013. The application is currently being reviewed by the EMA.
Orexigen received approval for Contrave from the US FDA as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults in September 2014.
Obesity
Excessive accumulated fat in the body may increase the risk of various diseases, especially heart disease, Type II diabetes, cancer, osteoarthritis and breathing difficulties.
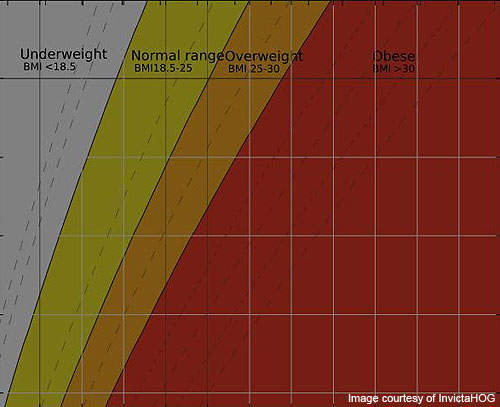
Obesity most commonly occurs due to lack of physical activity, excessive dietary intake and genetic reasons. It may also occur due to endocrine disorders and medication. Obesity can be determined through a Body Mass Index (BMI) measurement.
The increasing prevalence of obesity in adults and children is causing great concern to the health authorities worldwide. According to CDC estimates, in the US alone about 34% of adults and 17% of children or teens are obese.
Contrave’s mechanism of action
Contrave is a combination of two previously approved drugs for other ailments. The two drugs used in Contrave combination are Bupropion, which is used for smoking cessation, and naltrexone, which is used against drug addictions.
The drug was tested with different combinations but the most effective combination so far has been 360mg of bupropion with 32mg of naltrexone.
Contrave is an anti-obesity drug which reduces appetite or inhibits fat absorption. The drug is available in tablet form for oral administration.
Clinical trials
Orexigen conducted Phase II clinical trials on Contrave from April 2005 to March 2007. The study enrolled 410 patients with the purpose of determining the safety and efficacy of the drug by using three different combinations of the drugs such with different doses and a placebo.
Orexigen has initiated four Phase III clinical trials on Contrave for the treatment of obesity.
In April 2007, the company initiated a 56-week COR-BMOD Phase III clinical trial to evaluate the safety and efficacy of Contrave alone. This trial enrolled 800 patients across nine centres.
In May 2007, a COR-Diabetes Phase III clinical trial focused on assessing the safety and efficacy of Contrave in obese subjects who also have been diagnosed with Type II diabetes. The study was initiated in 51 centres and enrolled 505 patients.
In October 2007, Orexigen started COR-I Phase III clinical trial on Contrave to assess its safety and efficacy in healthy, nondiabetic, obese patients. The study enrolled 1,742 patients. In July 2010, the company revealed the results from its 58-week COR-I Phase III trials.
The study showed that the patients who were administered with Contrave have lost body weight up to 5% to 10% when compared with placebo on both completers and intent-to-treat basis.
The key objective of COR (Contrave Obesity Research) was to evaluate the impact of weight loss on markers of cardiovascular and metabolic risk. The results from the COR-I trials showed that the drug has made significant improvements over placebo in insulin resistance, HDL cholesterol and cardiometabolic risk.
In December 2007, Orexigen started COR-II Phase III clinical trial which is a 56-week study designed to assess the safety and efficacy of Contrave. The study enrolled 1,496 patients.
The results of the trial were released in April 2011. It was observed that the patients maintained a normal circadian pattern of blood pressure variation over one year of the treatment. The results showed a 7.5% weight loss compared to just 2.5% weight loss observed in the placebo-administered patient group.
Data from the Ambulatory Blood Pressure Monitoring sub-study also showed weight loss and improvements in markers of cardiometabolic risk.
The FDA approval for the Contrave was based on the results obtained from four Phase III clinical trials named COR-I, COR-II, COR-BMOD and COR-Diabetes. The studies were conducted on 4,536 patients who were randomised to Contrave or placebo to evaluate the effectiveness of Contrave for one year.
The results of the four studies showed that non-diabetics treated with Contrave showed an average weight loss of 4.1% over treatment with placebo at one year.
The clinical trial programme also includes an ongoing study called LIGHT, which is a double-blind, placebo-controlled cardiovascular study that has enrolled 8,900 patients. The primary endpoint of the study is to evaluate the rate of major adverse cardiovascular events in overweight and obese adults with cardiovascular risk factors and receiving Contrave. The marketing authorisation in Europe will be based on the outcome of the LIGHT study.
Marketing commentary
In September 2010, Orexigen sold the US, Mexico and Canada marketing rights of the experimental Contrave drug to Japan-based company Takeda Pharmaceutical for $50m. Takeda plans to launch Contrave in the North American market in Fall 2014 and in the European market in early 2015.
On 12 March 2010, Orexigen entered into an exclusive manufacturing agreement with Patheon for the development of Contrave and future formulations of Orexigen products.
In June 2009, Orexigen obtained non-exclusive rights on certain formulation patents related to bupropion from GlaxoSmithKline.