CXL-1020 is an investigational therapeutic agent being developed for treating patients with acute decompensated heart failure. The drug is being developed by US based Cardioxyl Pharmaceuticals.
In April 2010, the company has started recruiting participants for Phase IIa clinical trials on the drug. The final results of the trials are expected to come by February 2011.
Acute decompensated heart failure
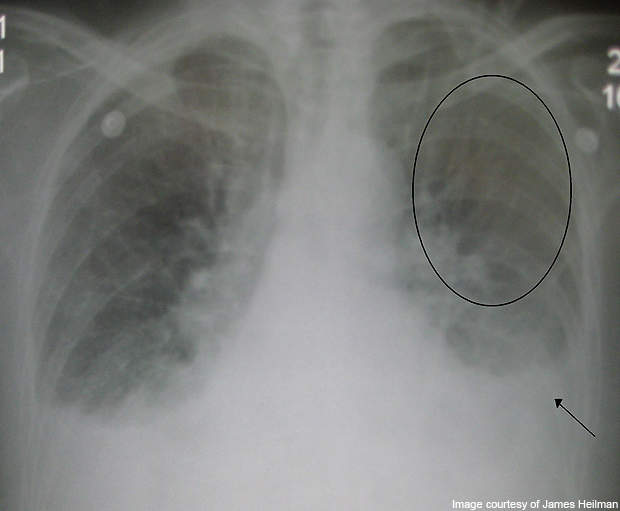
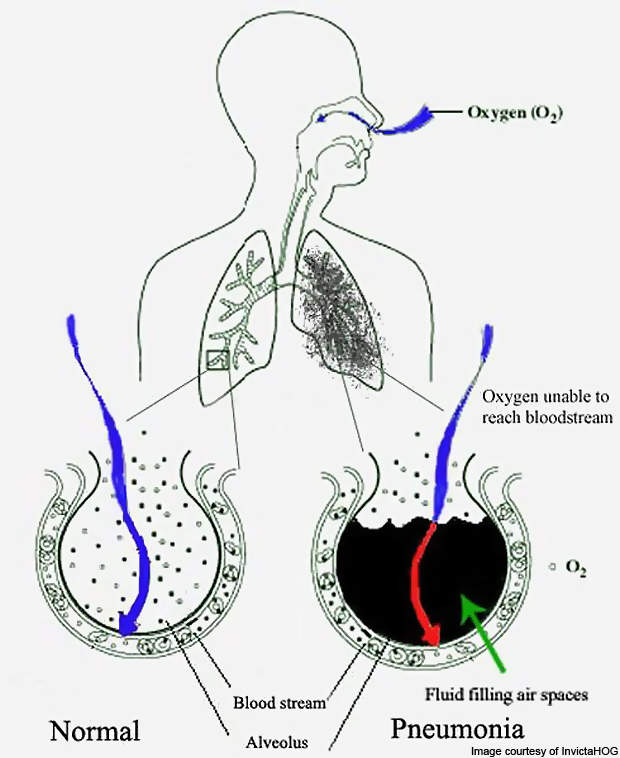
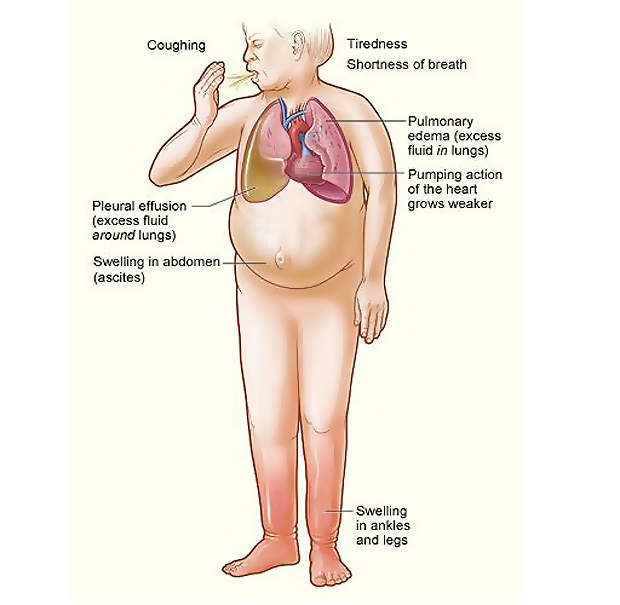
Acute decompensated heart failure is a cause of uncomfortable respiratory distress. ADHF is marked by severe reduction in the cardiac function, which affects the accumulation of fluid in the lungs and consequently results in shortness of breath. ADHF patients are often hospitalised.
It is a fatal condition with in-hospital mortality rates of 2% to 6% and 6-month readmission rates as high as 30% to 60%. ADHF most commonly may result from conditions such as a heart attack, hypertension, pneumonia, or it may also occur as a result of a patient’s failure to maintain a proper diet or take necessary medication.
It was estimated that in the US more than $39bn was spent on medical care of heart failure patients in 2009. In 2006, more than 1.1m acute heart failure hospitalisation cases were registered in the country.
Among the ADHF patients the 30-day mortality rate was 11% and 1 year mortality rate was 34%. According to the American Heart Association estimates there are approximately 4.9m people in the US alone who are living with congestive heart failure, 2.5m of them are males and 2.4m are females.
It has also been estimated that 10 out of every 1000 people above the age of 65 face this condition.
CXL-1020
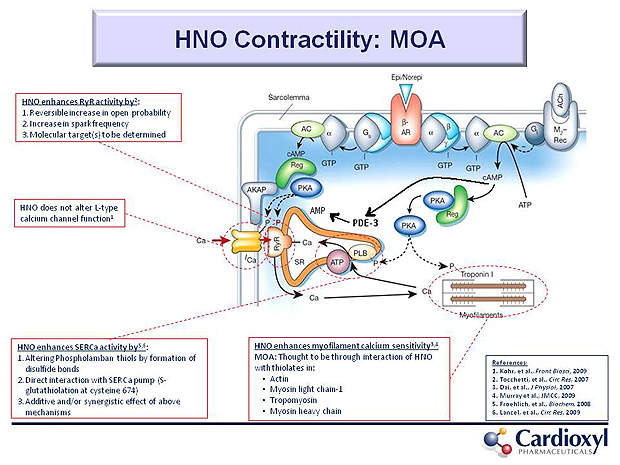
CXL-1020 has been developed on the nitroxyl chemistry platform for the treatment of ADHF. The drug is administered via intravenous infusion and contains nitroxyl that shows positive direct myocardial and vasodilatory effects.
In the preclinical studies conducted on CXL-1020 it was found that the drug can improve cardiovascular performance by increasing the inotropy and lusitropy of the failing heart.
Clinical trials
In 2007, Cardioxyl initiated pre-clinical development of CXL-1020 for treating ADHF. The company completed the pre-clinical development of the drug in 2008.
Cardioxyl initiated Phase I/II clinical trials on CXL-1020 in June 2009 and concluded them by July 2010. The study enrolled 28 people with stable heart failure, who were administered with three doses of CXL-1020 intravenous infusion. It was found that the drug had accepted levels of 10ug/kg/min which the patients could tolerate well.
The safety and pharmacokinetic profile of the drug was acceptable too. During the study the drug had not led to any adverse or serious events. Moreover, the drug demonstrated statistically substantial hemodynamic activity in patients with relevant underlying disease.
Cardioxyl started Phase IIa clinical trials on the drug in April 2010. They are expected to conclude by February 2011. The company is currently recruiting patients for the study. The study is being conducted across 20 US and international clinical sites and is designed to further define an appropriate clinical dosage for the drug.
The study will enrol 66 participants with decompensated heart failure. The study will evaluate hemodynamic parameters in intravenous infusion of the drug at several dose levels.
It will also evaluate safety information of the drug and dose-related hemodynamic effects.
Marketing commentary
Once it is approved the demand for CXL-1020 will be immense as there are no first-line therapies available currently to treat ADHF. Hospital admissions for ADHF have increased in the recent times and are projected to continue to increase in the future.
According to a study conducted by Center for Medicare and Medicaid Administration, ADHF was the most costly hospital diagnosis with more than $3.6bn spent in 1998.