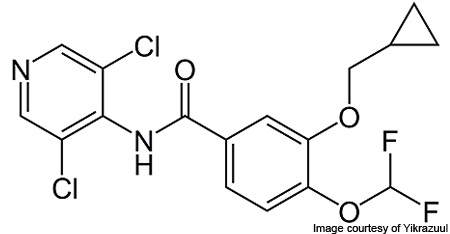
Daliresp (Roflumilast) is an oral tablet that is proved to reduce the risk of exacerbations in patients suffering from severe chronic obstructive pulmonary disease (COPD). The drug has been developed by Nycomed.
Marketed as Daxas in the European markets, Daliresp was approved by the US FDA in March 2011. The European marketing approval for Daxas was received in 2010.
Daliresp will be available in US markets in the second quarter of 2011. It will be marketed by Nycomed’s partner Forest Laboratories.
Chronic obstructive pulmonary disease
COPD is a progressive and irreversible lung disease which narrows the airways, thus causing respiratory problems. Breathlessness, chronic cough and congestion of phlegm in the lungs are the major symptoms associated with COPD.
These symptoms worsen further to cause exacerbation that lasts for weeks, requiring immediate hospitalisation and intensive medication. If left unattended, exacerbation can result in failure of lungs and could even be fatal.
Daliresp
Daliresp is an oral tablet to be taken once daily. It is the first and the only selective phosphodiesterase-4 (PDE4) inhibitor approved by the FDA.
Although the mechanism of action of the drug is not known, it is believed that the therapeutic action is exerted in patients by effects related to increase in intracellular cyclic adenosine metaphosphate (cAMP) in lung cells.
Clinical trials
Daliresp was evaluated in eight clinical trials conducted in a randomised double-blind, controlled parallel group. Around 9,394 patients of 40 years and above were enrolled in these trials.
Trials 1 and 2 were placebo-controlled dose selection trials of six months duration. These trials were conducted on 1,929 patients to evaluate the efficacy of Daliresp 250mcg and 500mcg tablet once daily. The median age of the enrolled patients was 63 years, associated with severe COPD in the range of 30%-80%.
Of the total randomised population, 751 patients were administered with 250mcg Daliresp and 724 with 500mcg. The 500mcg was selected over 250mcg based on the nominal improvement in lung function.
Trials 3, 4, 5 and 6 were once-year placebo-controlled trials. These were conducted to evaluate the efficacy of the treatment in patients who reported to have less than 50% of COPD severity and faced exacerbations. The enrolled patients were in the median age of 64 years.
Trials 3 and 4 included patients with less than 50% severity with chronic bronchitis and smoking history of 10 years. Trial 3 enrolled 1,176 patients of whom 567 were tested with Daliresp. Trial 4 was conducted on 1,514 patients of whom 760 were treated with Daliresp.
Both these trials failed to show significant reduction in the exacerbations in majority of the population. However, exploratory studies revealed that the treatment showed better response in reduction of COPD exacerbation in a small section of the population. Therefore further trials 5 and 6 were conducted on patients associated with chronic bronchitis, at least one COPD exacerbation in the previous year and a 20 pack year of smoking history.
Trial 5 had enrolled a total of 1,525 patients of whom 765 were administered Daliresp. Trial 6 had a total of 1,571 patients enrolled of whom 772 were tested with Daliresp. In both the trials, 500mcg Daliresp once daily confirmed that the treatment of Daliresp had resulted in a significant reduction of moderate or severe exacerbations compared to placebo. These two trials proved the safety and supported the use of Daliresp in the reduction of exacerbations of COPD.
Trials 7 and 8 were six months trails conducted to evaluate the efficacy of Daliresp as an add-on therapy to a bronchodilator. Patients enrolled for these trials were with moderate to severe COPD and of 65 years of median age.
The trials were conducted on patients with moderate to severe (40%-70% predicted) COPD without any chronic bronchitis or history of exacerbations. The results indicated that the drug showed significant response in reducing the exacerbations.