Duaklir Genuair (aclidinium bromide / formoterol fumarate) is an inhalation powder indicated as a maintenance bronchodilator treatment for chronic obstructive pulmonary disease (COPD).
Discovered by Almirall and developed by AstraZeneca, the drug was granted marketing authorisation (MA) by the European Commission (EC) as a maintenance bronchodilator treatment for adult COPD patients in November 2014. The MA is applicable in all member states of the EU and the European Economic Area.
AstraZeneca and Circassia Pharmaceuticals formed a strategic collaboration in March 2017 to submit a new drug application (NDA) for Duaklir Genuair in the US, which is expected to be submitted in the first half of 2018. Circassia will have the rights to market Duaklir Genuair in the US, as part of the collaboration.
Chronic obstructive pulmonary disease (COPD)
Onbrez capsules are hard capsules developed by Novartis for the treatment of COPD.
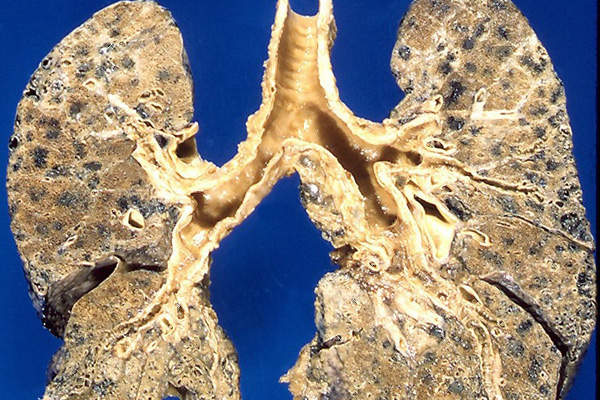
COPD is a progressive disease associated with various lung conditions, including chronic bronchitis, emphysema and chronic obstructive airways.
These diseases limit the flow of air to and from the lungs, causing patients to experience difficulty breathing. COPD is mainly associated with tobacco smoking, air pollution and occupational exposure.
It is estimated that COPD affects approximately 300 million people worldwide and will be the third leading cause of death by 2020.
Duaklir Genuair’s mechanism of action
Duaklir Genuair is a combination of long-acting bronchodilator drugs aclidinium bromide (approved under brand name Eklira) and formoterol fumarate. Both share a similar pharmacodynamic profile but exhibit different mechanisms of action (MoA).
Aclidinium bromide is an anticholinergic or long-acting muscarinic antagonist (LAMA) that inhibits the muscarinic M3 receptor to produce a bronchodilation effect.
Formoterol fumarate is a long-acting beta agonist (LABA) that produces bronchodilation by stimulating β2 receptors in the bronchial smooth muscle. Both drugs were individually approved for maintenance treatment of COPD in the US and Europe.
Compared to individual therapies, twice-daily therapy with Duaklir Genuair showed significant improvement in COPD patients.
Drug administration
Duaklir Genuair is administered orally through the Genuair dry powder inhaler device, which contains a dry powder formulation of aclidinium bromide and formoterol fumarate. The device delivers 340mcg of aclidinium bromide and 12mcg of formoterol fumarate per actuation.
The Genuair inhaler is a multi-dose oral inhaler pre-loaded with doses required for one month’s treatment. It is specially designed for COPD patients and comes with a unique combination of optical and acoustic feedback systems.
The device makes a click when activated and a coloured window on the side changes from green to red when the patient inhales correctly. The inhaler is said to deliver a consistent dose independent of the patient’s respiratory efforts.
In addition, the device incorporates a visible dose indicator showing an approximate number of remaining doses, an anti-dosing mechanism, and an end-of-dose lock-out system, preventing the use of an empty inhaler.
Clinical trials on Duaklir Genuair
AstraZeneca plans to submit an NDA for Duaklir Genuair based on data from the Phase III Amplify study. This 24-week, multi-centre, randomised, double-blind and parallel-group study assessed the safety and efficacy of aclidinium bromide / formoterol and tiotropium in moderate-to-very-severe stable COPD patients.
Announced in September 2017, results from the study indicated that patients treated with Duaklir Genuair showed statistical improvement in lung function.
EU approval of Duaklir Genuair was based on results from Phase III clinical trials conducted to discover the safety and efficacy of the drug. The studies enrolled 2,000 patients in 11 clinical trials conducted in 29 countries.
Studies showed that Duaklir Genuair demonstrated significant and sustained improvement in the lung function of patients, compared to monotherapy. In Phase III trials, bronchodilator effects were seen within five minutes of first administration and were continued over the dosing interval.
A Phase III clinical development programme on Duaklir Genuair enrolled approximately 4,000 COPD patients. It included two randomised, control and active-controlled studies known as Acliform (LAC 30) and Augment (LAC 31), which were conducted for six months to investigate the use of aclidinium / formoterol fumarate combination in the treatment of moderate-to-severe COPD.
The programme also included a six month extension of the Augment study (LAC 36) and a long-term 12-month randomised controlled study, which compared aclidinium / formoterol 340 / 12 to formoterol (LAC 32).
In the Acliform study, the combination drug demonstrated improvements in FEV1 one hour after the dose compared to a placebo and aclidinium of 299ml and 125ml. It also showed improvements in trough FEV1 compared to a placebo and 143ml and 85ml of formoterol.
In the Augment study, the combination demonstrated improvements in FEV1 after one hour of first dose compared to a placebo and aclidinium of 284ml and 108ml, as well as improvements in trough FEV1 relative to placebo and 130ml of formoterol.
The drug showed significant improvement in symptoms such as breathlessness with an improved baseline transition dyspnea index (TDI) focal score at six months compared to placebo. TDI was 1.29 units in the Acliform study and 1.44 units in the Augment study. Approximately 64.8% in the Acliform study and 58.1% in the Augment study achieved improved TDI scores compared to placebo.
Pooled analysis of both studies showed that Duaklir Genuair achieved significant improvements in TDI focal scores compared to placebo and other individual therapies.
In addition, the combination drug successfully improved daily symptoms of COPD such as breathlessness, chest symptoms, coughing and sputum, as well as symptoms limiting early morning activities and overall night-time and early morning symptoms compared to placebo, aclidinium and formoterol.