Enjaymo™ (sutimlimab-jome) is the first and only approved treatment for adults suffering from cold agglutinin disease (CAD), a rare blood condition. The drug addresses a severe and chronic unmet medical need for CAD patients.
Developed by French multinational healthcare company Sanofi, the drug reduces the need for red blood cell transfusions in CAD patients due to the destruction of red blood cells (haemolysis).
Enjaymo is supplied as a colourless to slightly yellow, preservative-free solution containing 1,100mg/22ml (50mg/ml) of sutimlimab-jome in a single dose vial for intravenous use.
Regulatory approvals for Enjaymo
In March 2020, Sanofi submitted a biologics license application (BLA) for Enjaymo to treat haemolysis in adult patients with CAD to the US Food and Drug Administration (FDA). The BLA was accepted under priority review in May 2020.
The FDA granted breakthrough therapy and orphan drug designations to Sutimlimab. The drug was also given orphan drug designation by the European Medicines Agency (EMA) and Japan’s Pharmaceuticals and Medical Devices Agency.
In November 2020, Sanofi received a complete response letter from the FDA regarding the BLA, which identified certain inadequacies related to a third-party production facility. The company resubmitted the BLA to the FDA in August 2021 and it was accepted for review the following month.
The FDA approved Enjaymo for the treatment of adults with CAD in February 2022.
Cold agglutinin disease causes and symptoms
CAD is a rare autoimmune haemolytic disease characterised by the destruction of red blood cells (RBCs). It can result in anaemia and cold-induced circulatory symptoms such as discomfort and discolouration of the fingers or toes.
The condition is known as cold agglutinin disease because RBC death occurs at low temperatures. It is caused when antibodies called cold agglutinin bind to the surface of RBCs. This triggers a process in which the body’s immune system attacks healthy RBCs, causing them to burst.
Patients with CAD suffer from severe anaemia, which can lead to tiredness, weakness, shortness of breath, light-headedness, chest discomfort, irregular heartbeat and other potential complications since RBCs deliver oxygen throughout the body. Many CAD patients require RBC transfusions to manage their condition.
The rare and chronic condition affects around one in every million people a year and primarily affects people aged between 40 and 80 years. It is estimated to affect 5,000 individuals in the US.
Enjaymo’s mechanism of action
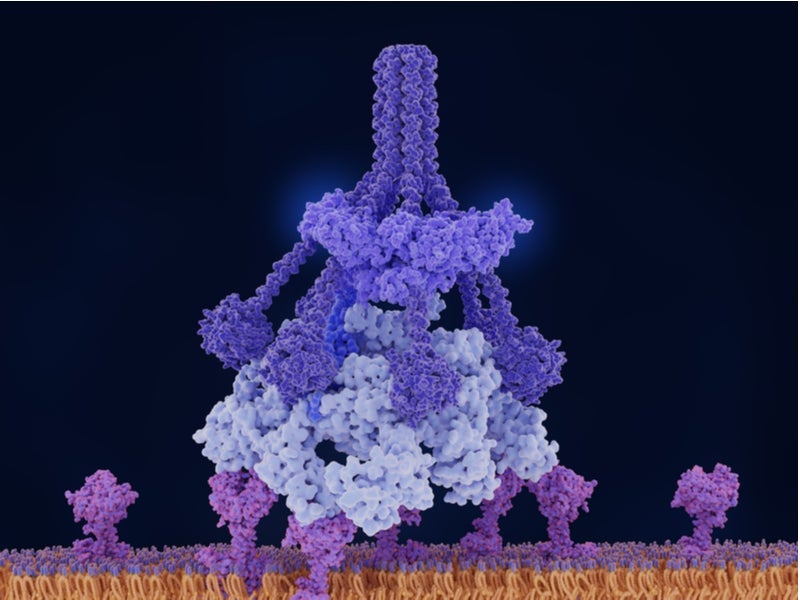
Enjaymo is a humanised monoclonal antibody that works by preventing haemolysis or RBC damage. The drug is designed to specifically target and inhibit complement protein component 1s subcomponent (C1s), which is part of the immune system’s classical complement pathway.
Enjaymo inhibits the activation of the complement cascade in the immune system and C1-activated haemolysis in CAD, thereby preventing healthy RBCs from being destroyed. The drug does not affect the functional activities of alternative or lectin complement pathways, while inhibiting the classical complement pathway.
Clinical trials on Enjaymo
The FDA approval for Enjaymo was based on the positive outcomes of a 26-week, single-arm, six-month pivotal Phase III CARDINAL clinical trial.
The trial assessed the efficacy of Enjaymo in 24 patients with CAD who had recently received blood transfusions.
The patients received a 6.5g or 7.5g injection of Enjaymo for 25 weeks based on their body weight. All patients received Enjaymo for up to six months and had the option of continuing treatment subsequently in the trial.
Enjaymo’s efficacy was measured based on the primary endpoints of a 2g/dL increase in haemoglobin from baseline or reaching a haemoglobin level of 12g/dL at the 26-week treatment evaluation time point, as well as the absence of transfusions or CAD treatments from week five to 26.
Results from the trial showed that 54% of patients achieved the primary endpoints, with 63% reaching a haemoglobin level of 12g/dl or an increase of at least 2g/dl, while 71% were transfusion-free after week five and 92% were not using further CAD-related therapies.
The drug also met the secondary endpoints of haemoglobin improvement and bilirubin normalisation. The average recorded reduction in bilirubin levels was 2.23mg/dl from a baseline level of 3.23mg/dl.
The most common side effects reported with the use of Enjaymo were diarrhoea, cough, dyspepsia, viral infection, swelling in the lower legs and hands, arthralgia, and arthritis.