Erleada™ (apalutamide) is an androgen receptor inhibitor indicated for the treatment of patients with non-metastatic castration-resistant prostate cancer (NM-CRPC).
The drug was discovered and developed by Johnson & Johnson’s subsidiary Janssen.
Janssen submitted a new drug application (NDA) for Erleada™ to the US Food and Drug Administration (FDA) in October 2017 and received priority review designation. The FDA approved the drug in February 2018.
Erleada™ was approved in Canada as the first treatment for men suffering from NM-CRPC in June 2018.
Janssen submitted a marketing authorisation application (MAA) for the drug to the European Medicines Agency (EMA) in February 2018.
Prostate cancer symptoms and causes
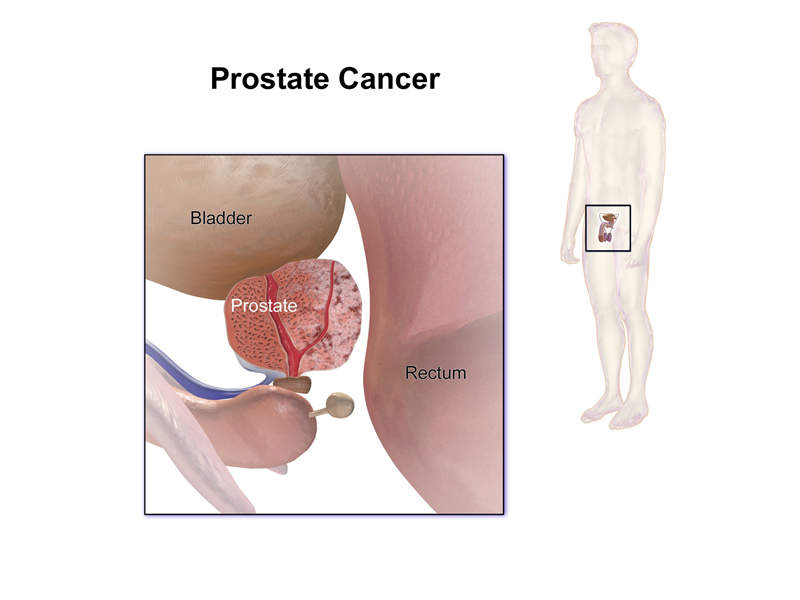
Prostate cancer is one of the most common cancers and originates in the prostate gland.
The disease has no signs or symptoms in the early stages, but may cause difficulties urinating, a decreased stream of urine, discomfort in the pelvic area, blood in semen, erectile dysfunction and bone pain in advanced stages.
In the NM-CRPC stage, the disease does not respond to medical or surgical treatments. An estimated 90% of patients with castration-resistant prostate cancer will develop bone metastases that can lead to pain, fractures and spinal cord compression. The survival rate for patients with distant stage prostate cancer is approximately 30%.
According to the American Cancer Society’s estimates, 161,360 new cases of prostate cancer were reported in 2017. Of these, 26,730 resulted in death.
Erleada mechanism of action
Erleada™ contains an androgen receptor (AR) inhibitor that directly binds to the ligand-binding domain of the AR. It restrains the nuclear translocation of the AR and DNA binding and obstructs AR-mediated transcription.
The drug is available in 60mg tablets for oral administration.
Clinical trials on Erleada
The FDA’s approval for Erleada™ was based on a Phase III clinical trial named SPARTAN. This randomised, double-blind, placebo-controlled, multi-centre study enrolled 1,207 NM-CRPC patients who had rapidly rising prostate-specific antigens (PSA) and were on continuous androgen deprivation therapy.
The study was conducted in 332 sites located across 26 countries in North America, Europe, the Asia-Pacific (APAC) and Australia. Patients were randomised in 2:1 ratio to receive Erleada™ 240mg once daily in combination with androgen deprivation therapy (ADT), or placebo once daily in combination with ADT.
All the patients enrolled in the study had previously received a concomitant gonadotropin-releasing hormone (GnRH) analogue or a bilateral orchiectomy.
The study results demonstrated that the risk of distant metastasis or death decreased by 72% in patients treated with Erleada™ compared with placebo. Median metastasis-free survival (MFS) was improved by more than two years in NM-CRPC patients with rising PSA, compared with placebo.
The MFS was 40.5 months for Erleada™ in combination with ADT compared with 16.2 months for placebo.
The results also showed that the time-to-metastasis (TTM) in the Erleada™ group was 40.51 months compared to 16.2 months for the placebo group. The patients treated with Erleada™ also met other secondary endpoints of the study, including progression-free survival (PFS) and time to symptomatic progression.
The most common adverse reactions reported in patients treated with Erleada™ included fatigue, rashes, nausea, weight loss, arthralgia, reduced appetite, fractures and peripheral oedemas.