Evusheld (previously AZD7442) is a new combination therapy comprising two long-acting antibodies (LAABs), tixagevimab and cilgavimab, for pre-exposure prevention of the coronavirus disease 2019 (Covid-19) in high-risk adults and children aged 12 years and older.
Discovered by Vanderbilt University Medical Centre in 2020, the drug was licensed to AstraZeneca under a licensing agreement for optimisation and clinical development in June 2020. AstraZeneca moved the new antibody combination to a Phase III clinical trial in January 2021.
Evusheld is the only Covid-19 antibody administered intramuscularly (150mg tixagevimab and 150mg cilgavimab). A single dose is administered via two separate injections. Additional doses of the drug for prolonged protection are determined and administered by healthcare providers once every six months.
Regulatory approvals for Evusheld
In October 2021, AstraZeneca submitted a request for emergency use authorisation (EUA) of AZD7442 to the US Food and Drug Administration (FDA) for Covid-19 prophylaxis. The FDA granted EUA to Evusheld (tixagevimab and cilgavimab) for the condition in December 2021.
In January 2023, the FDA revised the EUA limiting the drug’s use in cases when the national prevalence of all non-susceptible SARS-CoV-2 variants is less than 90%. As a result, the drug is not authorised for use in the US. Clinical trial data showed that the drug is inactive against certain SARS-CoV-2 variants, indicating that it is not expected to offer protection against contracting Covid-19.
Evusheld received provisional approval from the Therapeutic Goods Administration (TGA), Australia for the treatment and prevention of Covid-19 in January 2023.
In 2022, Evusheld was authorised by the Medicines and Healthcare products Regulatory Agency (MHRA) for the prevention of Covid-19 in the UK. The National Institute for Health and Care Excellence (NICE) issued a draft guidance after the US revised EUA for Evusheld in February 2023. The guidance stated that Evusheld is not recommended for use in vulnerable adults who are at high risk of contracting severe Covid-19.
Evusheld was recommended by the Committee for Medicinal Products for Human Use (CHMP) and approved by the European Medicines Agency (EMA) in March 2022.
In August 2022, Japan’s Ministry of Health, Labour, and Welfare granted special approval for Evusheld Emergency for adults and adolescents based on the findings of the TACKEL trial.
Covid-19 causes and symptoms
Covid-19 is caused by the SARS-CoV-2 virus and can be contracted by close contact with another infected person.
The disease’s severity ranges from no recorded or very minor symptoms to severe sickness, possibly resulting in death. Although Covid-19 illness is usually mild, it can cause significant complications and exacerbate other medical conditions.
People of all ages with severe, chronic illnesses such as heart disease, lung disease and diabetes appear to be at greater risk of being hospitalised with Covid-19.
Symptoms of Covid-19 include diarrhoea, congestion or runny nose, fatigue, nausea, headache, loss of taste or smell, sore throat, cough, fever or chills, muscle or body aches and shortness of breath or difficulty in breathing.
Evusheld’s mechanism of action
Evusheld (tixagevimab co-packaged with cilgavimab) is a combination monoclonal antibody therapy generated from B-cells donated by patients who have recovered from SARS-CoV-2 infection.
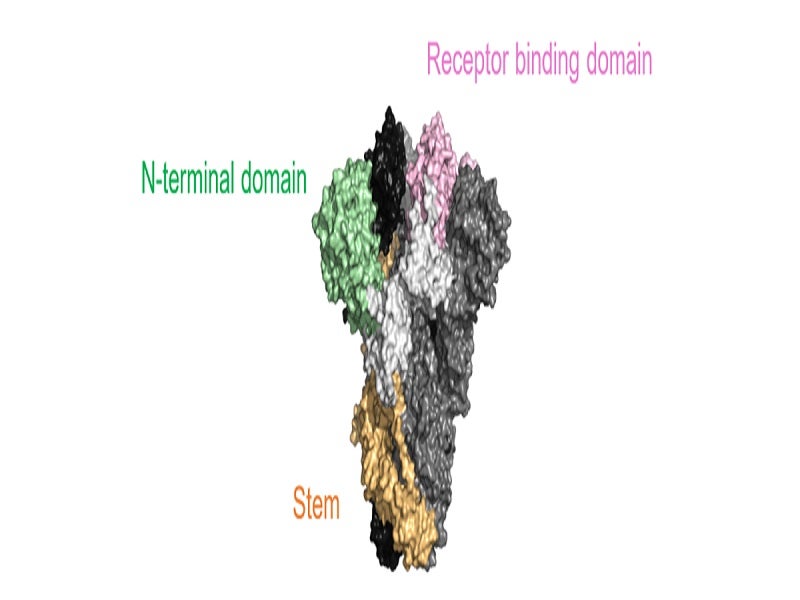
The spike protein on the SARS-CoV-2 virus is the primary target being explored for the development of Covid-19 monoclonal antibody therapies. The spike protein allows the virus to bind to and fuse with the host cell membrane.
AstraZeneca has optimised human monoclonal antibodies to bind to distinct parts of the receptor-binding domains located on the SARS-CoV-2 spike protein. In this way, the drug neutralises the virus’ capacity to infect healthy cells with reduced Fc receptor protein and complement component 1q (C1q) protein complex binding.
The reduced Fc receptor protein binding intends to minimise the possibility of antibody-dependent enhancement of illness, a condition in which virus-specific antibodies promote infection or disease rather than preventing it.
The half-life of the monoclonal antibodies has also been extended using proprietary YTE half-life extension technology, with the aim of extending their longevity in the body for long-term protection.
Evusheld has demonstrated effectiveness in neutralising recent SARS-CoV-2 variants, including the Delta and Mu variants. The drug also demonstrated effective neutralising activity against the Omicron variant in an independent study conducted by the FDA.
Clinical trials on Evusheld
The FDA’s EUA for Evusheld was based on the results of a randomised, double-blind, placebo-controlled, multi-centre, Phase III pre-exposure prevention clinical trial named PROVENT.
The trial enrolled 5,197 adult participants to evaluate the safety and efficacy of a single dose of AZD7442 compared with placebo for Covid-19 prevention. The participants were randomly assigned at a 2:1 ratio to receive either 300mg Evusheld or saline placebo in two separate, sequential intramuscular injections.
The study’s primary endpoint was the first incidence of any SARS-CoV-2 RT-PCR positive symptomatic illness development post-dose before day 183.
The trial showed a 77% lower risk of developing symptomatic Covid-19 in patients administered with Evusheld compared with those given placebo.
A six-month assessment of data from the study demonstrated that participants administered with AZD7442 had an 83% lower risk of contracting symptomatic Covid-19 than those given placebo.
Possible adverse effects of Evusheld include hypersensitivity reactions (including anaphylaxis), haemorrhage at the injection site, headache, fatigue and cough.
Evusheld was also investigated in the TACKLE Phase III randomised, double-blind, placebo-controlled, multi-centre trial conducted to compare the safety and efficacy of a single 600mg IM dose of Evusheld with placebo for the outpatient treatment of mild-to-moderate Covid-19.
The results of the trial showed that Evusheld significantly reduced the relative risk of progressing to severe Covid-19 or death by 50% compared to placebo.