Exanta (ximelagatran) is an oral Direct Thrombin Inhibitor (DTI) under development by AstraZeneca. As the first fixed-dose, oral anticoagulant to be approved since the introduction of warfarin over 50 years ago, it was seen as an important new treatment for the prevention and treatment of Venous Thromboembolism (VTE) and potentially for stroke prevention in patients with atrial fibrillation.
Exanta works by inhibiting the action of thrombin in the coagulation cascade. Thrombin converts the soluble protein fibrinogen to soluble fibrin in the final stage of blood coagulation. By inhibiting the action of thrombin, Exanta prevents clot formation.
AstraZeneca filed for drug approval in Europe and US in 2003, subsequently gaining approval in France for VTE prevention in patients undergoing orthopaedic surgery. Since filing was under the EU’s mutual recognition procedure this approval extended to all EU countries. However, the UK and Ireland were unable to agree with the product label and withdrew from the mutual recognition process. Although approval in the US was widely anticipated it failed to materialise when in 2004 the FDA rejected Exanta on the recommendation of its cardiovascular and renal drug’s advisory committee.
In March 2006, AstraZeneca took the decision to withdraw Exanta from the market on the grounds of safety and terminate further development of the drug.
VENOUS THROMBOEMBOLISM (VTE) IN SURGERY
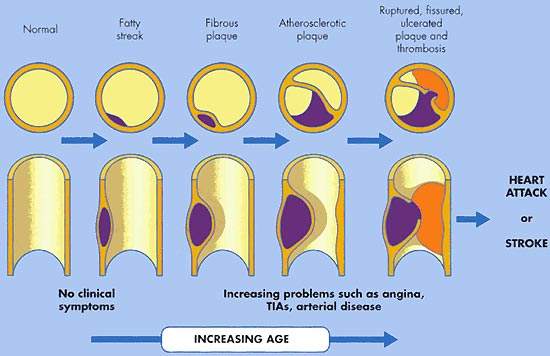
VTE encompasses both deep-vein thrombosis (DVT) and pulmonary embolism (PE). It is the third most common cardiovascular disease after heart attack and stroke. In the US alone, it is estimated that 2 million people are affected by VTE. Of these, 60,000 die from PE, which occurs when a clot in the deep veins of the leg breaks away and becomes lodged in the pulmonary artery. VTE is often asymptomatic.
Patients undergoing major orthopaedic surgery (hip or knee replacements, hip fracture) are at an especially high risk of developing VTE. Without anticoagulant therapy, between 40% and 50% of patients undergoing hip replacement surgery suffer VTE. This rises to 70% to 80% in hip fracture surgery.
EXANTA IN THE PREVENTION OF VTE
The efficacy and safety of Exanta in the prevention of VTE in patients undergoing major orthopaedic surgery has been investigated in a series of large-scale phase III trials.
The European EXPRESS (EXpanded PRophylaxis Evaluation Surgery Study) trial in 2,800 patients showed that in at-risk patients undergoing elective hip or knee replacement surgery, pre-operative administration of subcutaneous melagatran (Exanta parent compound) followed by oral Exanta provided superior VTE prophylaxis compared with enoxaparin, the current gold standard.
The US EXULT (Exanta Used to Lessen Thrombosis) trial in 2,301 patients, in which Exanta was compared with warfarin for VTE prophylaxis following knee replacement surgery, showed the incidence of VTE was 20.3% in the Exanta group compared with 27.6% for those on warfarin.
Exanta’s superior efficacy profile compared with standard anticoagulant therapies (enoxaparin/warfarin) is accompanied by a good safety profile with respect to clinically significant bleeding. In the THRIVE III (Oral Direct THRombin Inhibitor ximelagatran for Venous ThromboEmbolism) trial, long-term administration of Exanta to 1,223 patients with prior VTE was well tolerated and associated with low bleeding rates. In this trial the estimated cumulative risk of recurrent VTE was 2.8% for Exanta compared with 12.6% for placebo-treated patients, a statistically significant difference. Recent data from the longer-term THRIVE Treatment study have confirmed these findings.
EXANTA IN THE PREVENTION OF STROKE
Stroke prevention remains an area of significant unmet clinical need. The potential to use Exanta in stroke prevention in patients with atrial fibrillation is being explored in a series of clinical trials, including SPORTIF III (Stroke Prevention Oral Thrombin Inhibitor in Atrial Fibrillation) and SPORTIF V.
Results from the SPORTIF III trial showed that a twice-daily dose of Exanta 36mg compared favourably with dose-adjusted warfarin, in preventing stroke and systemic embolic events in patients with atrial fibrillation. Although not designed to demonstrate superiority, the incidence of stroke and systemic embolic events was numerically lower in the Exanta treatment arm (40 events vs. 56 on warfarin). A 29% relative risk reduction favouring Exanta was observed in respect of stroke and other systemic embolic events. Overall, 4.6% of Exanta-treated patients experienced the composite 12-month endpoint of stroke, major bleeding or death, compared with 6.1% of warfarin-treated patients.
Results of the SPORTIF V trial, presented at the American Heart Association meeting in November 2003, were consistent with the earlier SPORTIF III results. Again, no significant differences were observed between treatments with respect to any of the primary endpoints, such as stroke or systemic embolism. While rates of major bleeding were similar in both groups, combined rates of major and minor bleeding were significantly lower with Exanta than with warfarin.
MARKETING COMMENTARY
For the millions of people who are at-risk of or experience thromboembolic diseases (VTE, stroke and myocardial infarction) each year there is a need for better anticoagulant therapies. Estimates suggest that at present only one in two patients with atrial fibrillation receives an anticoagulant for stroke prevention and yet these patients are at a five-fold greater risk of stroke.
Although eligible patients will no longer have access to Exanta following its recent withdrawal for safety reasons, AstraZeneca remains committed to the development of oral anticoagulants. Development work on a related compound, AZD0837, continues.