Fabhalta (iptacopan) is a factor B inhibitor approved for treating adults with paroxysmal nocturnal hemoglobinuria (PNH), a rare and chronic blood disorder.
Novartis, a pharmaceutical company based in Switzerland, developed the drug.
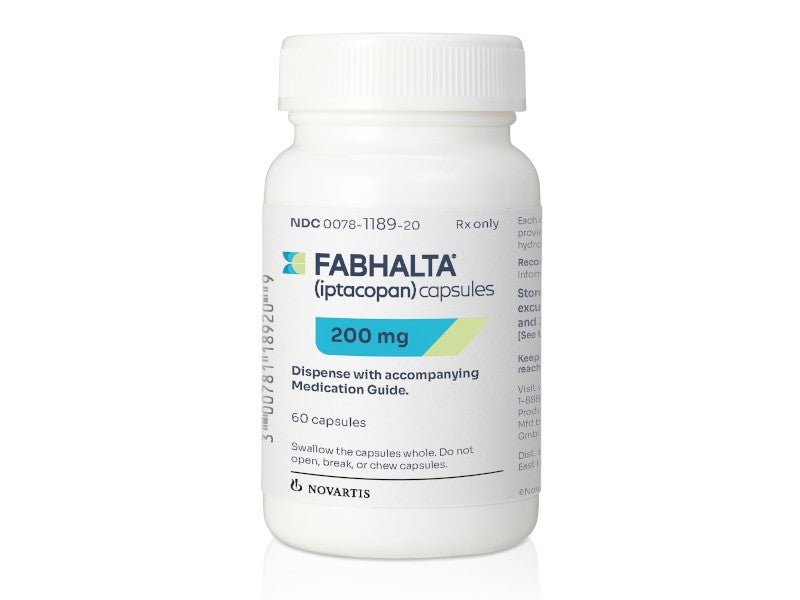
Fabhalta is available as a pale yellow, opaque, hard gelatin capsule, each containing 200mg of iptacopan for oral administration.
Regulatory approvals for Fabhalta
Fabhalta was approved by the US Food and Drug Administration (FDA) in December 2023 as a monotherapy for treating adults with PNH.
The FDA granted Iptacopan breakthrough therapy designation for PNH treatment and rare paediatric disease (RPD) designation for C3 glomerulopathy (C3G) in December 2020.
Additionally, in October 2020, the European Medicines Agency (EMA) granted iptacopan orphan drug designation for IgA nephropathy (IgAN) and the PRIority MEdicines (PRIME) designation for C3G.
The PRIME scheme, introduced by the EMA, aims to support the development of novel drugs that fulfil unmet medical needs.
PNH causes and symptoms
PNH is a rare disease caused by an acquired genetic mutation of the phosphatidylinositol glycan biosynthesis class A gene (PIGA) in the hematopoietic stem cells.
The hematopoietic stem cells develop into red blood cells (RBCs), white blood cells and platelets within the bone marrow. A mutation in the hematopoietic stem cells causes the RBCs to be destroyed prematurely by a person’s immune system, known as the complement system.
PNH can lead to the destruction of RBCs in the blood vessels in a process called intravascular haemolysis (IVH) and the destruction of RBCs in the spleen and liver, called extravascular haemolysis (EVH). It can cause haemolytic anaemia, chronic kidney disease, thrombosis and other conditions, if untreated. Typical symptoms include shortness of breath, the presence of haemoglobin in the urine, fatigue and abnormally pale skin.
It is estimated that approximately ten to 20 people per million live with PNH globally. The condition is diagnosed in those aged between 30 and 40, with women more likely to be affected.
Mechanism of action
Iptacopan binds to Factor B, which is a key component of the alternative complement pathway (AP). It inhibits the activation of the AP C3 and C5 proprotein convertases and prevents the downstream cell destruction and activation of the terminal pathway.
Iptacopan controls both C3b-mediated EVH and terminal complement-mediated IVH.
Clinical trials on Fabhalta
The FDA’s approval of Fabhalta was based on data from a pivotal Phase III international, multicentre, randomised, active-comparator, open-label clinical trial named Apply-PNH.
The study was conducted to demonstrate the efficacy and safety of Fabhalta by evaluating the superiority of the Fabhalta switched group to anti-C5 therapy in patients with residual anaemia after prior anti-C5 treatment.
The study enrolled 97 patients who were randomised in an 8:5 ratio to receive either 200mg of iptacopan as a monotherapy or anti-C5 therapy intravenously.
Results of the study demonstrated a substantial increase in haemoglobin levels, with 82.3% of the patients who switched to Fabhalta experiencing an increase of more than 2g/dL and 67.7% achieving more than 12g/dL increase, all without the need for red blood cell transfusions, as compared to the anti-C5 treatment group.
Approval for Fabhalta was also supported by data derived from the APPOINT-PNH multicentre, open-label, uncontrolled single-arm extended study. The APPOINT-PNH study aimed to assess the efficacy and safety of Fabhalta in PNH patients who are naïve to complement inhibitor therapy, including anti-C5 therapies.
The study enrolled 40 patients with PNH who received Fabhalta 200mg as a monotherapy. Results of the study showed that continuous Fabhalta treatment enabled most patients to achieve clinically meaningful increases in haemoglobin levels of 2g/dL or more from baseline, without the need for blood transfusions.
Prevalent adverse reactions observed during the clinical trials were headache, nasopharyngitis, diarrhoea, abdominal pain, and bacterial and viral infections.