Gaboxadol is a direct-acting gamma-amino butyric acid (GABA)A agonist under development by Danish pharmaceutical company Lundbeck for the treatment of sleep disorders. It has a different mode of action from benzodiazepine ligands, mediating its effects via a GABA receptor population that is not modulated by benzodiazepines.
Gaboxadol has now entered phase III development for the treatment of insomnia, the most common sleep disorder, following promising results from phase II trials.
GABOXADOL HAS POTENTIAL TO INCREASE TREATMENT OPTIONS FOR SLEEP DISORDERS
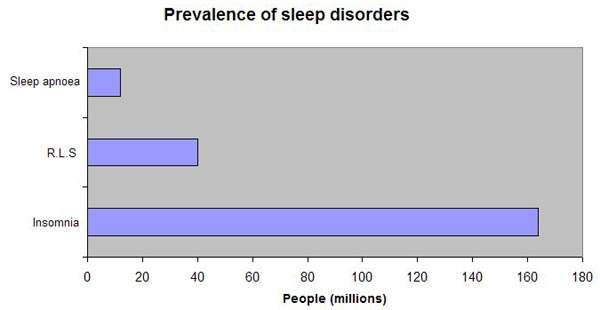
Estimated to affect between 20% and 30% of the general population, sleep disorders are extremely common. Treatments range from self-medication with non-pharmaceutical preparations to prescription medications, such as benzodiazepines, antidepressants, antihistamines and, most recently, selective hypnotics. Although the new generation of selective or dedicated hypnotic agents represent a major advance in the treatment of insomnia, they still carry some risk of dependence. Ideally, drug therapy for insomnia should induce sleep that is qualitatively close to normal sleep cycles and be devoid of rebound and withdrawal effects.
Phase II studies, in which gaboxadol has been studied in patients with primary insomnia, suggest it is effective with respect to classical sleep parameters as well as with respect to Slow Wave Sleep, which is believed to influence the quality and restorative effects of sleep. A recently completed study in a transient insomnia model involving 100 subjects showed gaboxadol achieved statistically significant effects on both sleep induction and sleep maintenance. Importantly, these effects appear to be achieved without the risk of drug dependence.
Gaboxadol is now being evaluated in phase III trials involving around 100 investigational sites in Europe and Canada. The multi-centre, randomised, double-blind phase III trials, initiated in June 2003, are designed to evaluate the efficacy and safety of gaboxadol in 650 outpatients with chronic primary insomnia. Early treatment of primary insomnia is considered important because if left untreated it can become a chronic disorder that may lead to the onset of depressive states.
INVIVODATA’S ELECTRONIC PATIENT DIARY WILL BE USED TO RECORD SLEEP CYCLES IN TRIAL PARTICIPANTS
In the phase III trials patients will use a hand-held electronic diary to record the quality and duration of their sleep cycles as well as recording how long it takes them to fall asleep. The eDiary system used in the trials has been developed by invivodata of the US, a pioneer in the development of electronic patient self-reporting systems for clinical research. The invivodata eDiary system was selected following a rigorous competitive in-house pilot programme.
Sleep-related data entered by patients is fed via invivodata servers into a Web-based system that provides real-time reporting. The system enables continuous trial management by study investigators, which the companies believe will lead to high rates of patient compliance. Invivodata’s eDiary system is multilingual and asks patients about their sleep patterns in their native language. Feedback is then given on their data entries.
MERCK AND CO. TO CO-PROMOTE GABOXADOL IN THE US
Lundbeck recently signed an agreement with Merck and Co. to develop and commercialise gaboxadol in the US market, which constitutes some 65% to 75% of the global market for CNS drugs. This represents an important development for Merck, as it gives the company access to a potentially valuable CNS medication with approval anticipated sometime between the end of 2006 and mid-2007. Under the agreement the two companies will co-promote gaboxadol to psychiatrists in the US if it secures FDA approval.
In June 2004 Merck and Lundbeck extended their co-development and commercialisation agreement for gaboxadol to Japan. The companies will jointly conduct the development programme required for filing an NDA in Japan and will co-promote gaboxadol once it secures regulatory approval.
MARKETING COMMENTARY
In the US alone it is estimated that more than 40 million people suffer from chronic sleep disorders, while a further 20 million suffer occasional sleep problems. For years, conventional benzodiazepines were the mainstay of pharmacological treatment of insomnia. Gradually they have been replaced by the new selective hypnotics, which now dominate the market for drugs to treat sleep disorders and continue to drive its growth.
Sleep disorders remain poorly understood, under-diagnosed and under-treated. Good opportunities exist for new drugs to treat sleep disorders, especially where they offer benefits over existing agents. Lundbeck’s gaboxadol has a different mechanism of action to existing hypnotics and is believed to have minimal potential for abuse. This would be a major selling point once the drug is approved and gains market entry.